Introduction
Drug screening long involved making a compromise between obtaining functional information and paying for it with low throughput, or settling for limited affinity information but in high throughput.
High-throughput functional screening (HTS) has solved this conundrum by giving researchers the best of both worlds: the ability to go through huge libraries in a reasonable time and obtaining functional readouts. Functional readouts can, for example, eliminate time-consuming secondary assays by confirming the specificity of the ligand-target interaction and the binding of the ligand to the active rather than an allosteric site.
Performing high-throughput screens in droplet-based microfluidic systems is a way to further improve and miniaturize screening assays. Droplet-based systems result in order-of-magnitude improvements in compound consumption, reduce the need for capital equipment and enable single-cell analysis capabilities 1 - all without the loss of precision and the ability to automate2,3.
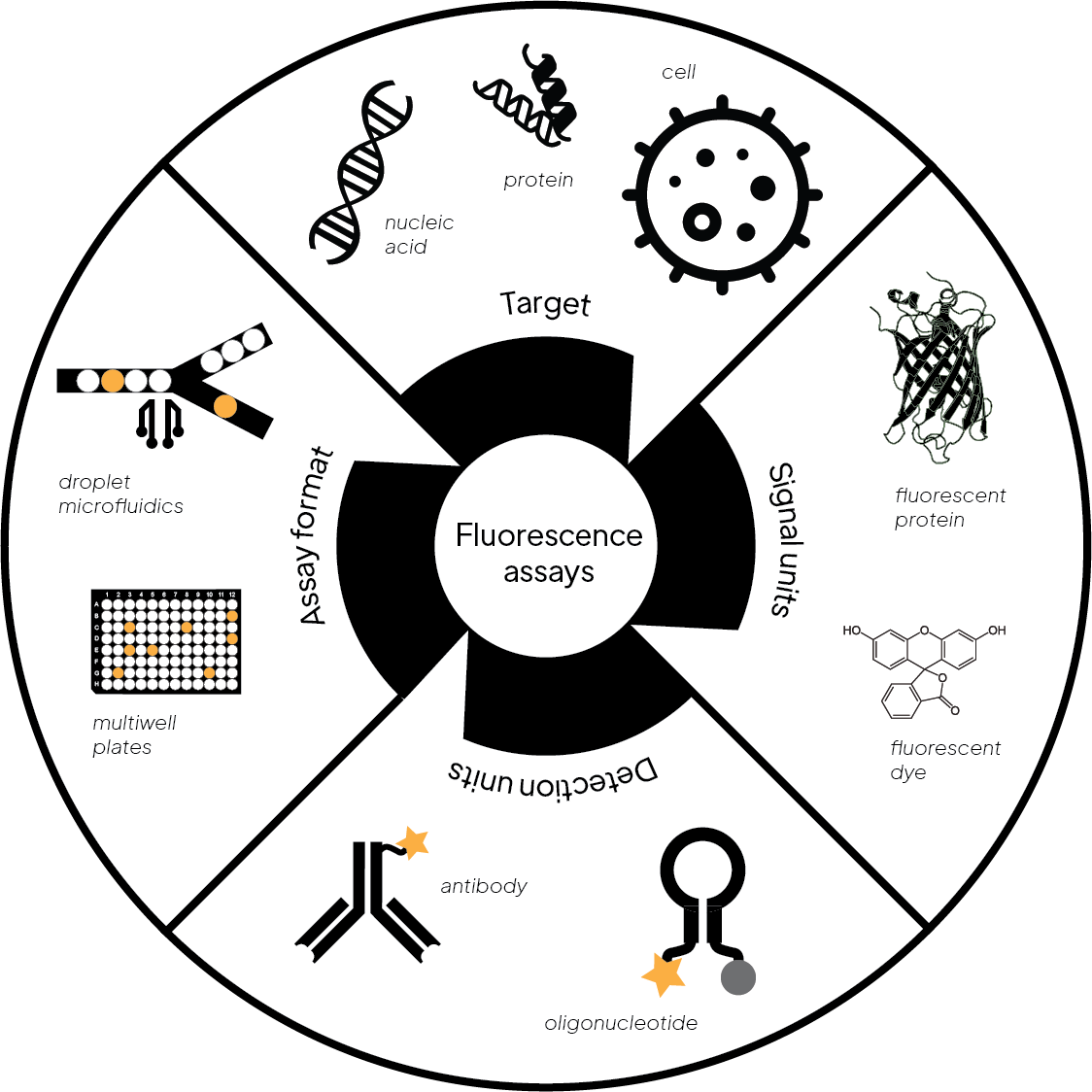
Figure 1. An overview of fluorescence assays
The Dominant Read-Out: Fluorescence
Fluorescence-based detection is the most used detection method in HTS for a number of reasons: it is highly sensitive with low background and resulting good signal-to-noise ratios. Fluorescence assays don't require the addition of detection reagents, can be miniaturized, and read out multiple times. In addition, fluorescence assays are amenable to different assay formats which makes them ideal tools for screening applications4.
A variety of assay designs exist that utilize fluorescent dyes for detection (Fig. 1). Here we summarize the most relevant ones for screening applications.
Fluorescence Intensity Assays (FLINT)
Fluorescent intensity assays measure the change of total light output and use it to quantify a reaction or binding event. The main types of FLINT assays are:
- ELISA assays are well-established immunoassays used to detect and quantify proteins and other analytes. While the original ELISAs used colorimetric readouts, fluorescent versions were developed for applications in HTS, e.g. quantifying analytes in pharmacokinetic studies and immunotherapeutic drug safety testing.
- Fluorogenic assays are used to measure enzymatic activity. The reactants are designed to be non-fluorescent, however, after cleavage by the enzyme, one reaction product is fluorescent and can be monitored by an increase in fluorescence intensity.
- Fluorescence quench assays use a fluorescent group in close proximity to a quencher. Enzymatic cleaving or conformational changes move the fluorophore and quencher apart leading to a quantifiable fluorescent signal.
- Fluorescent reporter gene assays are used to study the promoter of a gene by fusing it to a gene encoding for a fluorescent protein, e.g. GFP (green fluorescent protein). These assays are used to quantify the effect of an external stimulus, e.g. binding of a drug candidate, on the signaling cascade that ultimately leads to the expression of the fluorescent protein.
FLINT assays have many advantages: they are well-established, easy to perform and fairly cheap. However, they also have significant disadvantages, specifically, they are prone to fluorescent interference because many of the small molecules found in libraries used for screening fluoresce themselves.
Förster/Fluorescence Resonance Energy Transfer (FRET) Assay
FRET is a powerful detection method for identifying potential molecular interactions using a fluorescence readout.
FRET uses two fluorophores conjugated to the two biomolecules being studied, e.g. a ligand receptor pair. The donor fluorophore is excited by a light source and then transfers this energy to an acceptor fluorophore and both the loss of energy of the donor and the gain of energy of the acceptor can be measured. For the energy transfer to work, the donor and acceptor need to be in close proximity and the fluorophores need to have a good degree of spectral overlap.
FRET is ideally suited for HTS since it is simple, sensitive and easily automated and works well for a variety of possible detection techniques, incl. flow cytometry, immunocytochemistry, immunohistochemistry and ELISA.
Time-Resolved Fluorescence or Fluorescent Lifetime Assays
Time-resolved fluorescence (TRF) or fluorescence lifetime (FLT) is a widely used technique for measurements that are not based on intensity and therefore addresses the short-comings of fluorescence intensity measurements.
FLT measures the time a fluorophore spends in the excited state before it reverts to its ground state by emitting a photon. The fluorescent lifetime is characteristic for each fluorescent molecule but changes when a fluorescently-labeled molecule binds to an unlabeled component. These changes in FLT can be measured and quantified.
FLT assays are robust and highly useful for screening applications because the measurements are independent of the initial intensity and fluorophore concentration. They are also mostly unaffected by static quenching, fluorescent background from biological samples and light scattering.
The two main FLT techniques are time-correlated single-photon counting (TCSPC) and real-time decay curve analysis (RT-DCA). FLT can be combined with FRET, where the energy transfer between donor and acceptor leads to a change in fluorescence lifetime.
The drawback of lifetime measurements is that they only provide information about the kinetics of a reaction but no structural information about the reaction intermediates and by-products5.
Fluorescence Polarization (FP) Assays
Fluorescence polarization is suited for monitoring molecular interactions, e.g. protein-protein, protein-ligand, and protein-DNA binding, as well as enzyme activity in solution.
In FP, a fluorescent molecule is excited with plane-polarized light. During the time interval between excitation and fluorescent emission the dye molecule rotates due to Brownian motion and emits unpolarized light. FP measures that emission and consequently the rotation of the molecule. Since the rate at which the molecule rotates is a function of size, the smaller unbound dye shows a different FP than the dye bound to another molecule, e.g. an antigen.
FP is frequently used in competitive immunoassays formats where a fluorescently-labeled antigen competes with unlabeled antigen in solution.
Another advantage is that FP uses a single fluorescent label eliminating the need for additional separation and washing steps.
Designing Fluorescence Assays in Droplets
Fluorescence has also emerged as the most common read-out used in droplet microfluidics due to its bright signals and fast responses that are compatible with detecting sub-nanoliter droplet volumes at kilohertz rates6.
When designing fluorescence assays for a droplet format some important factors need to be considered.
Molecular Retention of Reaction Components
In order for droplets to serve as effective reaction vessels, they must retain all reaction components, incl. fluorophores for the duration of the reaction. This can be problematic as hydrophobic molecules tend to leak out of the droplets into the surrounding phase and eventually seep into other droplets. Leaking fluorophores impact assay sensitivity and increase false positive rates of fluorescence assays7.
Various approaches can address issues related to small molecule retention, e.g. modifications that add charges or the use of multiple surfactant layers.
Alternatively, addition of compounds that are known to increase droplet stability and retention, e.g. bovine serum albumin or sugars, to the aqueous phase is a viable option8.
Impermeability Makes Reagent Removal and Washing Challenging
One limitation of water-in-oil droplets is their impermeability: while various methods have been developed to add reagents, e.g. by merging droplets, they cannot be removed once the droplet is formed. Multi-step workflows that require reagent exchange, such as wash steps, are therefore challenging.
One way to address this issue is to use an aqueous two-phase system (ATPS) that is produced by liquid-liquid phase separation of polymers, such as polyethylene glycol (PEG) and dextran.
Designing no-wash assays, e.g. based on fluorescence polarization, provide another way of addressing impermeability challenges9.
Generating Unique Droplets
HTS requires each droplet to contain discrete components of the screening library but uniform reagent conditions. While this is straight-forward in well-plate based formats, there are significant challenges using droplets. Several approaches have been developed to enable the encapsulation of unique compounds such as combining multiple reagent streams prior to emulsification7.
Summary
Fluorescence assays (Fig. 2) dominate in high-throughput screening because of their high sensitivity, good tolerance to interference, fast signaling speed, high versatility, simplicity, and non-destructive way of tracking or analyzing targets10. Novel formats, such as droplet-based systems, pose new challenges that innovative approaches and careful assay design can address.
We have come a long way since the first immunofluorescent techniques were developed in the early 1940s and there is no end in sight: fluorescence assays are here to stay.
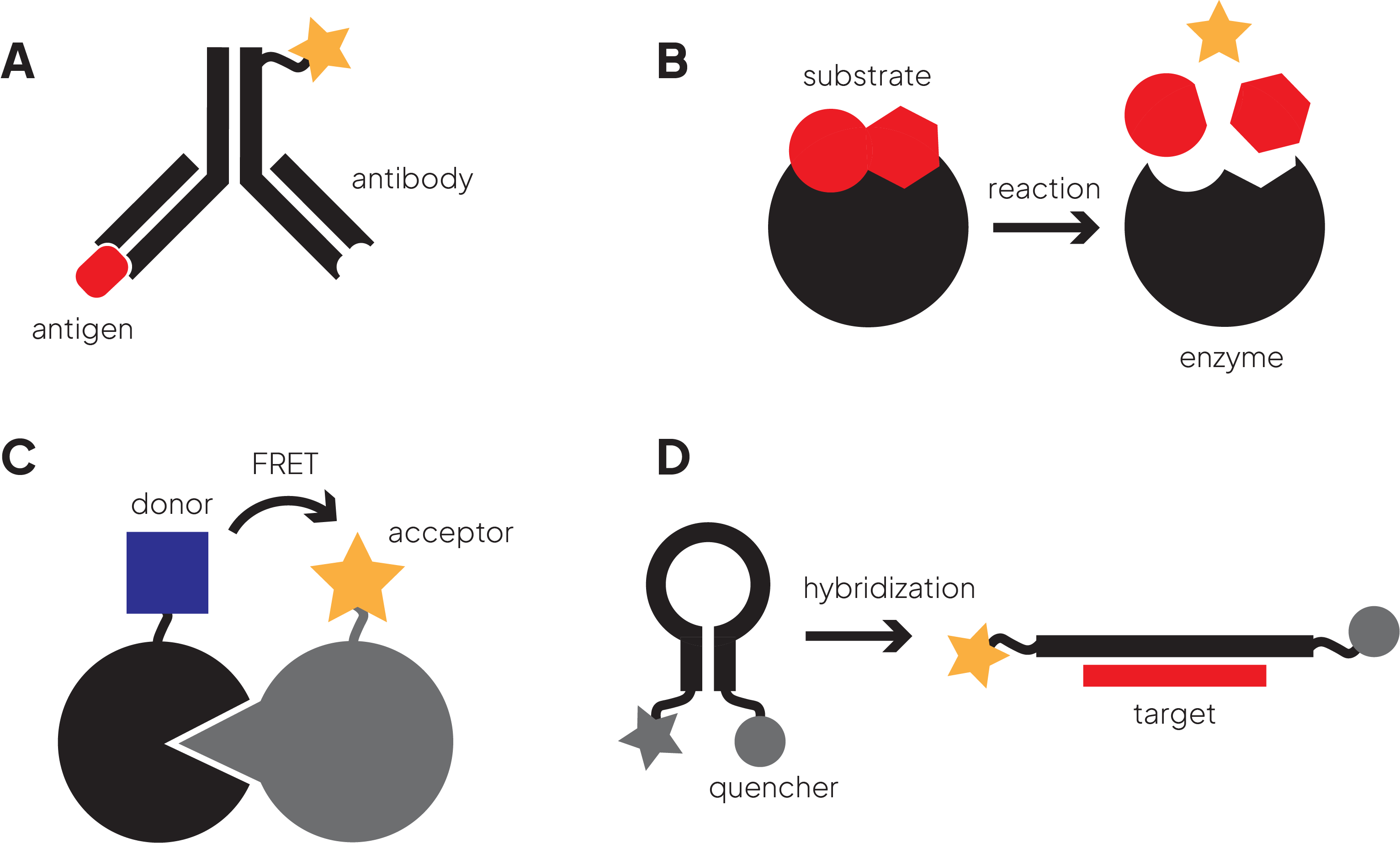
Figure 2. A variety of fluorescence-based assay designs
References:
-
Brouzes E, Medkova M, Savenelli N, Marran D, Twardowski M, Hutchison JB, Rothberg JM, Link DR, Perrimon N, SamuelsML. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. 2009;106 (34):14195-14200
-
Kerk YJ, Jameel A, Xing XH, Zhang C. Recent advances of integrated microfluidic suspension cell culture system. Engineering Biology. 2021;5(4): 81-97
-
Kulesa A, Kehe J, Hurtado JE, Tawde P, Blainey PC. Combinatorial drug discovery in nanoliter droplets. Proc Natl Acad Sci. 2018;115(26):6685-6690.
-
An WF. “Fluorescence-Based Assays” 2009 in Smith PA, Tolliday NJ, Wagner BK (eds) “Cell-Based Assays for High-Throughput Screening Methods and Protocols”, Humana Press:97-107
-
Kumar V, Schlücker S, Hasselbrink E. “Ultrafast time-resolved molecular spectroscopy” (2020) in Gupta VO, Ozaki Y (eds) “Molecular and Laser Spectroscopy Advances and Applications: Volume 2”, Elsevier Inc.: 563-594
-
Cole RH, Gartner ZJ, Abate AR. Multicolor Fluorescence Detection for Droplet Microfluidics Using Optical Fibers. J Vis Exp. 2016;(111):54010.
-
Payne EM, Holland-Moritz DA, Sun S, Kennedy RT. High-throughput screening by droplet microfluidics: perspective into key challenges and future prospects. Lab Chip. 2020;20(13):2247-2262
-
Price AK, Paegel BM. Discovery in Droplets. Anal Chem. 2016;88(1):339-53
-
Byrnes SA, Huynh T, Chang TC, Anderson CE, McDermott JJ, Oncina CI, Weigl BH, Nicols KP. Wash-Free, Digital Immunoassay in Polydisperse Droplets. Anal. Chem. 2020; 92(5): 3535–3543
-
Fang X, Zheng Y, Duan Y, Liu Y, Zhong W. Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening. Anal Chem. 2019 Jan 2;91(1):482-504.




