Drug delivery systems play a critical role in distributing therapeutic agents to targeted sites in the body for effective disease management. Nano- and microparticles have been engineered to carry drugs for their advantages such as increased drug stability, improved pharmacokinetics, and enhanced targeting capabilities. However, achieving uniformity and reproducibility using conventional batch synthesis has been a challenge. Droplet microfluidics has emerged as a promising technology in engineering particles for drug delivery applications due to its ease in producing monodispersed particles with immense control over their physical characteristics. Merging microfluidic chips with multiplexed functional assays has also enabled the evaluation of drug toxicity and efficacy. As a result, microfluidics-generated drug delivery systems hold great promise for improving therapeutic outcomes, minimizing side effects, and revolutionizing the field of drug delivery.
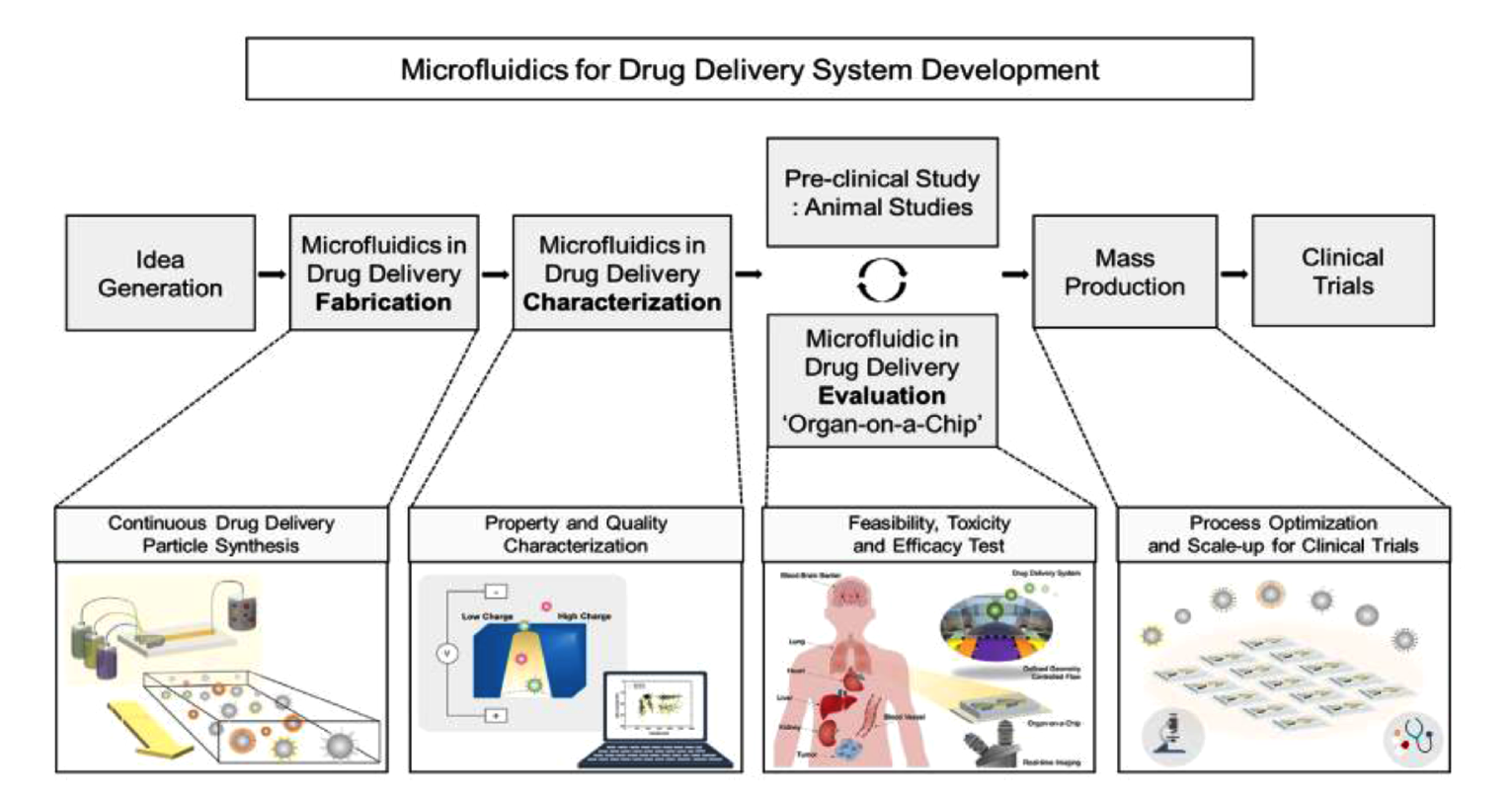
Traditional methods of particle fabrication often face challenges in controlling particle size and shape, which can impact various aspects of drug delivery including circulation time, cellular uptake, and release kinetics. In a recent publication, Al-wdan et al offer an overview of generating particles using microfluidics to overcome these hurdles. From the fabrication of drug-loaded particles to exact dosing, targeted delivery, and controlled release of drugs, microfluidic systems offer a comprehensive platform for drug delivery research and development. By manipulating fluid flow and mixing within microchannels, microfluidics produces particles of various sizes, shapes, and compositions to improve drug loading and pharmacokinetics. A wide range of materials, including lipid-based, polymer-based, and inorganic particles, can be fabricated using microfluidics. Biopolymers, such as chitosan, hyaluronic acid, and alginate, can be used to generate drug-loaded particles for their biocompatibility with host cells. Lipid-based particles, such as liposomes, are widely used due to their advantages in biocompatibility and high drug-loading capacity. Moreover, liposomes can encapsulate hydrophilic drugs within their aqueous core and lipophilic substance within their lipid bilayer. The ability to load multiple drugs with distinct physicochemical properties into the same particle enables the simultaneous delivery of different medications, facilitating combination therapy.
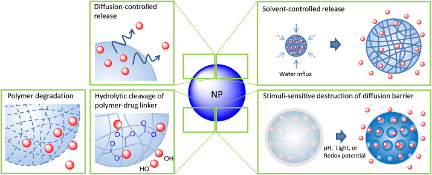
A fundamental aspect of drug delivery is controlling the release of therapeutics over time at targeted sites. Drug-loaded particles can be engineered to release drugs at a predetermined rate and location by modifying particle size, composition, and surface stability. One approach to achieving controlled release is the incorporation of stimuli-responsive materials into the particles. These materials can respond to external triggers such as changes in temperature, pH, or light, enabling drug release in a targeted and specific manner. By leveraging these responsive properties, drug delivery systems can enhance the selectivity and precision of drug release, leading to improved treatment outcomes and reduced side effects. Therefore, microfluidic devices provide a platform for the development of drug delivery systems that can release drugs in a programmable manner, mimicking complex dosing regimens required for specific diseases.

In one example, Kim et al examined the release of the anticancer drug doxorubicin (Dox) from pH-sensitive hydrogel microspheres. The authors hypothesized that encapsulating Dox in hydrogel microspheres would improve the diffusion of Dox in cancer cells, where the pH is typically more acidic. The hydrogel microspheres exhibited slower release of Dox at neutral pH due to the difficulty of hydrogel degradation at this condition. In acidic conditions (pH=4), the hydrogel microspheres degraded more easily, resulting in more effective drug release. Additionally, the solubility of Dox increased in acidic solutions, facilitating its release through diffusion. On the other hand, in basic conditions (pH=9), the release rate of Dox decreased due to reduced solubility. The pH-triggered release of Dox from hydrogel microspheres offers a promising approach for controlled drug delivery in cancer treatment, and further investigations are needed to explore their application in specific organs with animal models. Droplet microfluidics technology has greatly impacted the synthesis of drug delivery systems, opening new avenues to investigate novel drug encapsulation methods, optimize release profiles, and explore targeted medicine approaches.
- Al-wdan, O. A., Sharallah, O. A., Abdelwahab, N. A., Mohammed, A. O., Elmowafy, E., & Soliman, M. E. (2023). Insights into microfabrication and implementation of microfluidics in pharmaceutical drug delivery and analysis. OpenNano, 12, 100156.
- Lee, J. H., & Yeo, Y. (2015). Controlled drug release from pharmaceutical Nanocarriers. Chemical Engineering Science, 125, 75–84.
- Kim, D. I., Zhang, Y., Kim, H. H., Jeon, H. J., Kim, G.-C., & Go, J. S. (2014). Microfluidic synthesis of ph-sensitive multiamine hydrogel microparticles and release characterization of anticancer drug of doxorubicin (DOX). Journal of Drug Delivery Science and Technology, 24(5), 464–468.


