As monoclonal antibodies (mAbs) gain regulatory approval and widespread acceptance as a leading therapeutic option for the treatment of human diseases, there is a pressing need for new techniques and technologies that facilitate their rapid development. Droplet microfluidics technology is emerging as a powerful and agile tool for high-throughput antibody discovery, as thousands of individual mAb-producing cells can be compartmentalized within microdroplets and analyzed in parallel. Using picoliter- to nanoliter-sized droplets as reaction vessels also reduces reagent costs and ensures that detectable molecule concentrations are reached within short periods of time. Importantly, encapsulation within droplets retains the genotype-phenotype connection, even when the molecule of interest does not remain physically associated with the cell, as in the case of secreted antibodies. Droplet microfluidics technology also permits direct screening for function rather than merely revealing information about binding affinity, which can accelerate the screening campaign and increase its overall chance of success. Here, we showcase a droplet microfluidics-assisted approach for high-throughput screening and selection of antibody-producing cells using a Förster resonance energy transfer (FRET)-based detection assay.
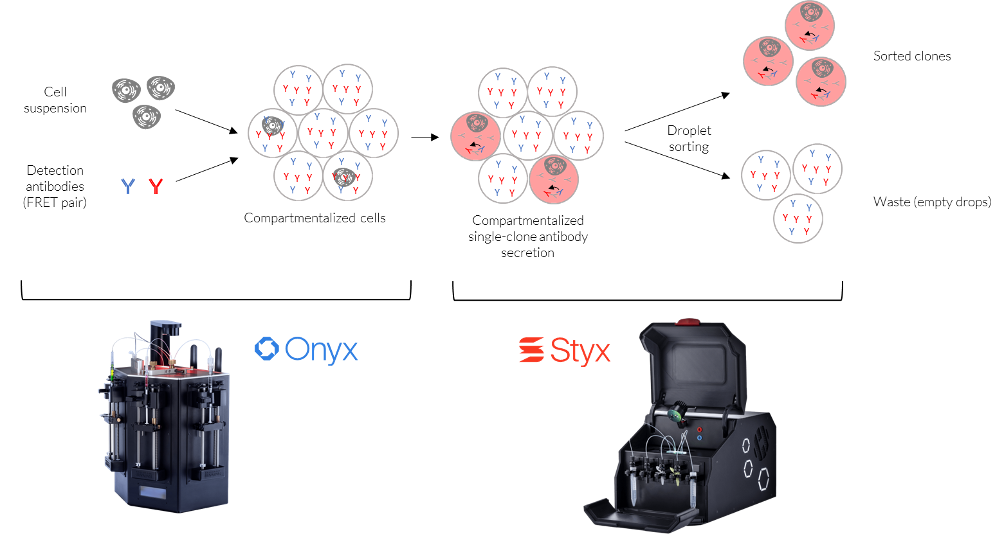
FRET-based detection assays identify molecular interactions, such as the binding of an antibody to an antigen, via the transfer of energy from a donor fluorophore to an acceptor fluorophore in close proximity to each other. To test a FRET-based assay in microfluidic droplets, a mixture of antibody-producing mouse hybridoma cells was encapsulated within droplets using the Onyx droplet generator. The cell concentration was adjusted based on the Poisson distribution, such that less than 1% of droplets would contain two or more cells. Two fluorescently labeled secondary antibodies, representing a donor-acceptor pair, were coencapsulated in all droplets, which ensured that each cell in the population could be analyzed. Antibodies produced by cells of interest will interact with the co-encapsulated detection antibodies, resulting in FRET between the two fluorescently labeled molecules upon excitation. In contrast, empty droplets or droplets containing cells that do not produce the antibody of interest will not result in a FRET signal. After encapsulation, the emulsion was incubated at standard cell culture conditions (37°C, 5% CO2) for 1 hour to allow antibody secretion.
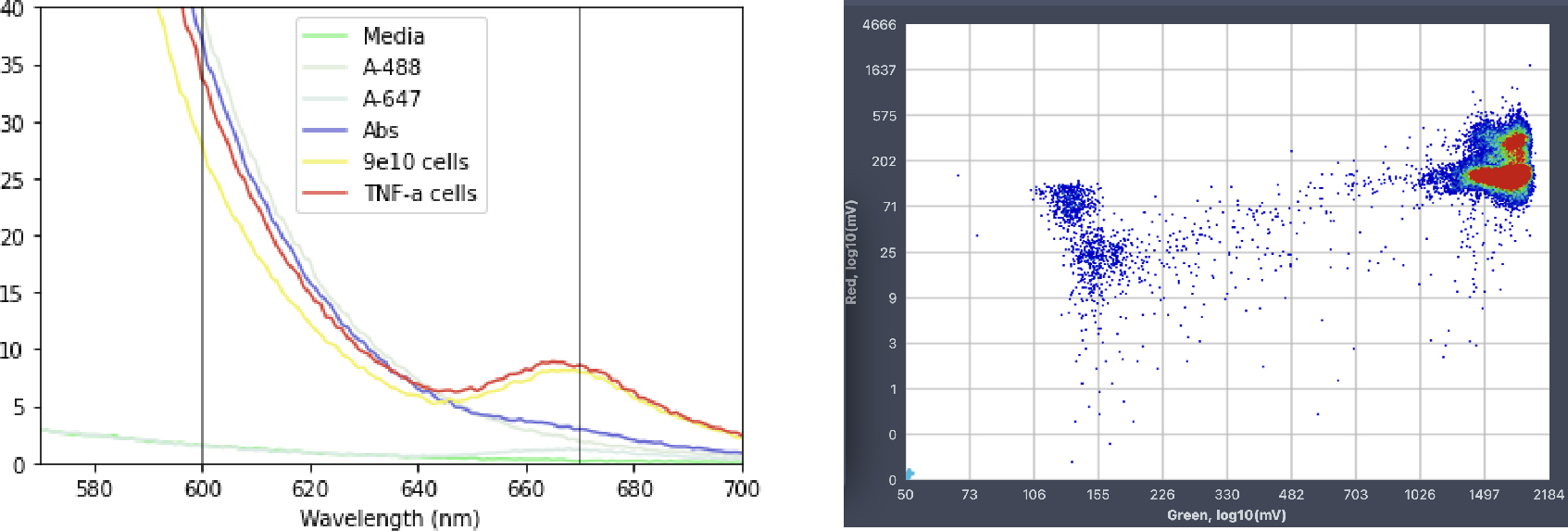
The FRET-based antibody detection system was first validated in a bulk spectrophotometric assay. As expected, a characteristic emission centered around 665 nm was only observed in the presence of all assay components and antibody-producing cells. To test the assay at the single-cell level, the droplet emulsion was reinjected into a sorting chip for high-throughput droplet analysis using the STYX system. Since only the green fluorescence was excited with a 488 nm laser, the appearance of increased red fluorescence indicated the FRET process and the presence of the antibody of interest. The results of droplet analysis on the STYX system were plotted on a scatterplot where the target droplet population can be selected manually, and the instrument will then set the sorting gates automatically. In this case, sorting gates were selected to capture the droplets exhibiting the increased red fluorescence.

A high-throughput fluorescence-activated droplet sorting (FADS) approach was employed via the Styx platform to select droplets containing antibody-producing cells, as indicated by increased red fluorescence. As opposed to high-throughput screening using fluorescence-activated cell sorting, FADS can be used to detect target molecules that are not physically associated with the cell, as is the case here with antibodies secreted by the cell. Indeed, droplet sorting using a FRET-based assay has been adapted to differentiate between and quantify cells expressing either membrane-bound or secreted antibody fractions. After sorting, the selected droplets were subjected to microscopy analysis, and the enrichment of droplets containing antibody-producing cells was confirmed. Thus, the use of the Onyx and Styx droplet microfluidic platforms enabled the isolation, detection, and selection of individual cells producing antibodies of interest based on FRET signal. Notably, this approach is applicable to high-throughput screening of both membrane-bound and secreted antibodies and can be adapted to provide information on both binding and functional activity.


