Functional screening is a selection and sorting strategy based on the activity of a target molecule in its biological context. Depending on the nature of the target molecule, functional screening assays can be designed around diverse readout principles, such as cell proliferation, expression of a reporter gene, downstream signaling, or other events that result from ligand binding. Functional screening is often preferred to screening based on binding affinity in many research areas, including drug discovery, functional genomics, and in vitro evolution, since affinity-based screening cannot directly reveal information about the target molecule’s functional activity.
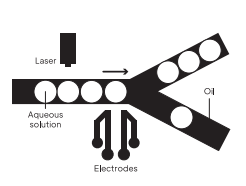
While screening throughput is a major bottleneck for traditional functional assays, droplet microfluidics technology can facilitate high-throughput functional screening with sorting rates as high as 30 kHz, screening millions of functional assays in hours compared to days. Fluorescence-activated droplet sorting (FADS) is the most mature on-chip droplet selection technique that sorts water-in-oil droplets based on a fluorescence readout. In FADS, droplets are introduced into a microfluidic chip with an asymmetric Y-shaped junction, where they will flow into the wider (waste) arm by default due to lower hydraulic resistance. If a fluorescent signal triggers a sorting event, an electrical pulse is applied to the sorting junction that deflects the droplet into the narrow arm of the sorting junction by dielectrophoresis. This sorting strategy is compatible with virtually any functional assay where activity can be revealed by a fluorogenic substrate.
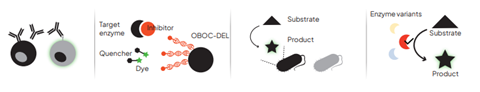
High-throughput FADS has the ability to accelerate functional screening workflows in many research areas. It can be used to perform phenotypic analysis of monoclonal antibody (mAb)-secreting cells, each individually coencapsulated with a reporter in its own reaction vessel, and can screen millions of these single mAb-secreting cells every day. It can be used to rapidly screen DNA-encoded libraries for molecules with a desired functional activity, facilitating early-stage drug discovery. Large metagenomic libraries can be rapidly screened to identify rare genes and activities in organisms, including unculturable bacteria. And FADS can be used to direct the in vitro evolution of enzymes with superior catalytic properties.
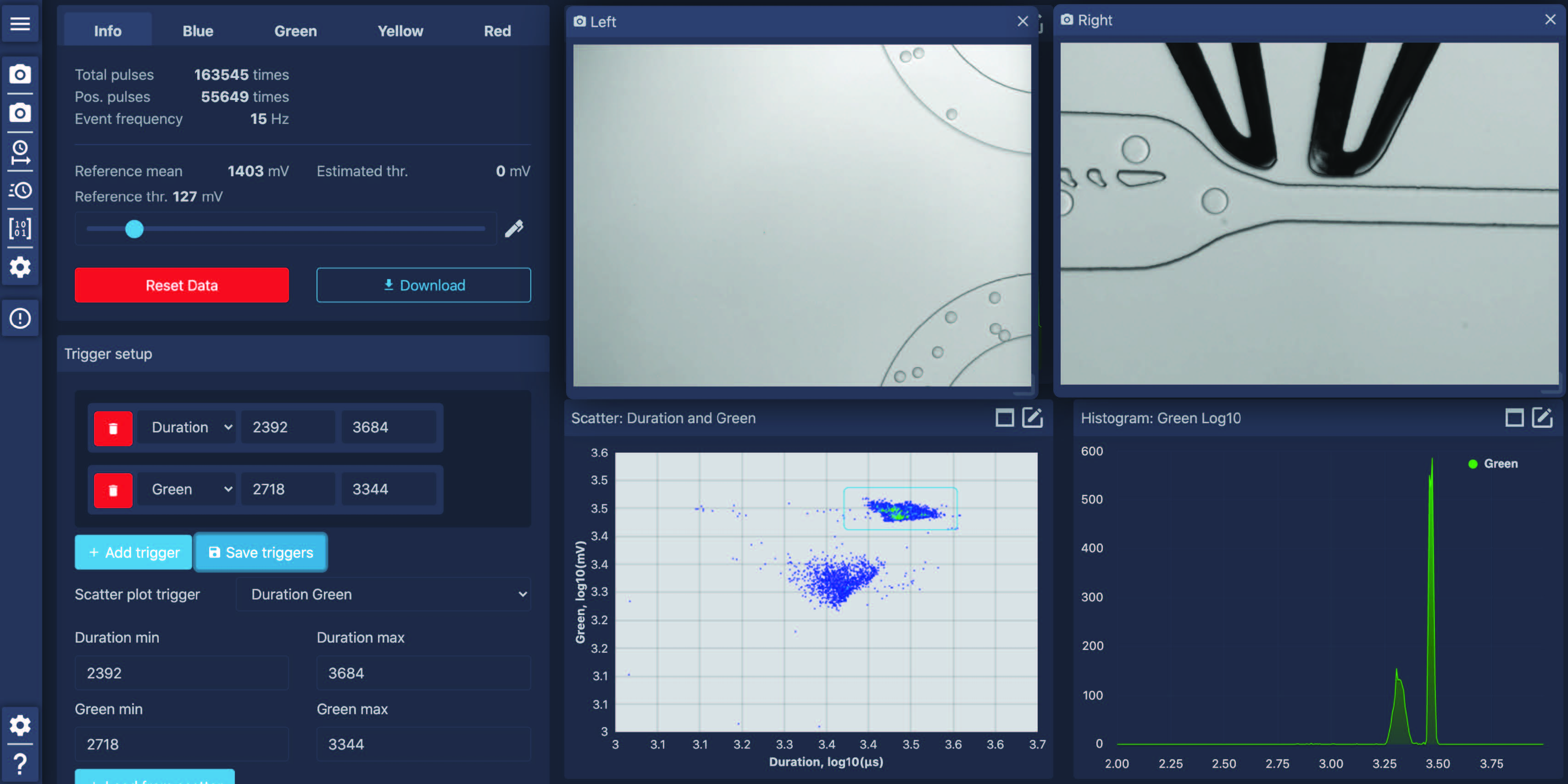
Droplet Genomics’ STYX platform is an all-in-one FADS platform designed for high-throughput functional screening. It enables you to perform any FADS functional assay, novel or published, and is fully compatible with any commercially available or custom-made elastomeric chip. Its easy-to-use software eliminates the technical complexity of FADS setup while retaining maximum design flexibility for any high-throughput screening campaign. Learn more about functional screening using STYX here.


