The inflammatory response of macrophages is a principal component of innate immunity that assists in the clearing of harmful stimuli, such as pathogens and irritants, from the body. The inflammatory response may also play a role in the development of certain diseases, such as cardiovascular disease, diabetes, cancer, and Alzheimer's disease, as well as contribute to the efficacy of vaccinations. However, the mechanisms of activation of this response have yet to be fully elucidated. Macrophages are known to trigger the inflammatory response through activation of the NLRP3 inflammasome, which releases the cytokine IL-1β and promotes cleavage of Gasdermin-D, resulting in inflammatory cell death (pyroptosis). Yet, questions remain as to exactly which viral surface antigens or other structural proteins may induce inflammasome activation. In this study, Lučiūnaité et al investigate how the structural properties of recombinant viral proteins impact NLRP3 inflammasome activation in macrophages. They also identify several macrophage subpopulations with divergent cell activation patterns, denoting the complexity of the inflammatory response.
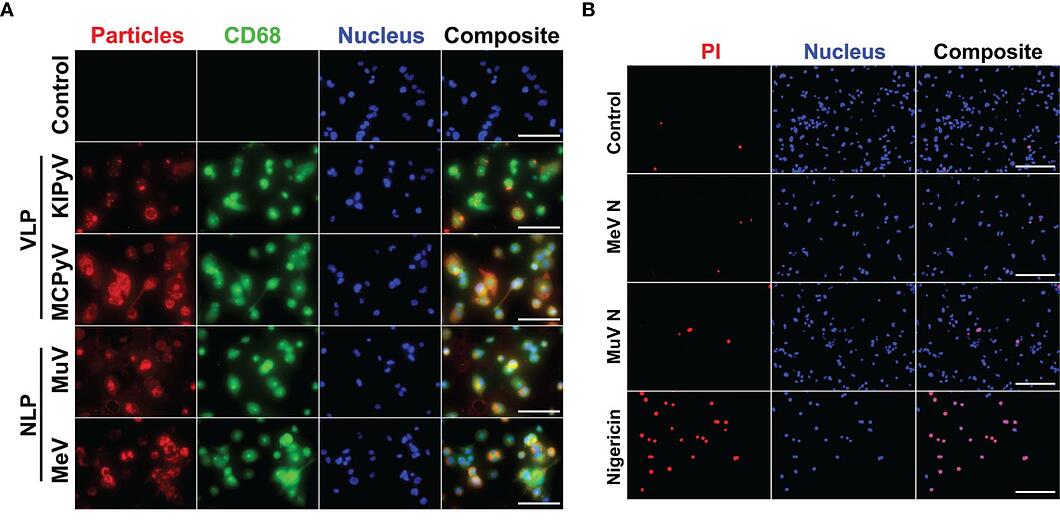
Two structurally diverse viral proteins were used for this study; these included the nucleocapsid-like particles (NLPs) of measles and mumps viruses, which form filamentous rod-shaped structures, and virus-like particles (VLPs) derived from capsid protein VP1 of human polyomaviruses, which form spherical structures. Human macrophage cell cultures were prepared from THP-1 cells and exposed to NLPs and VLPs for 24 hours. The macrophages were then immunostained with CD68 and anti-NLP and anti-VLP monoclonal antibodies to visualize the uptake of viral proteins. Both NLP and VLP recombinant viral proteins were shown to be taken up by THP-1 macrophages. However, an ELISA assay for human cytokines TNF-α and IL-1β revealed that only the spherical VLPs induced the inflammatory response. This result was recapitulated by cytotoxicity (lactate dehydrogenase release) and cell viability (propidium iodide and Hoechst nuclear staining) assays, where VLPs resulted in cell death and NLPs did not. The authors concluded that filamentous NLPs do not affect the inflammatory response and focused the remainder of their study on VLP-induced activation.
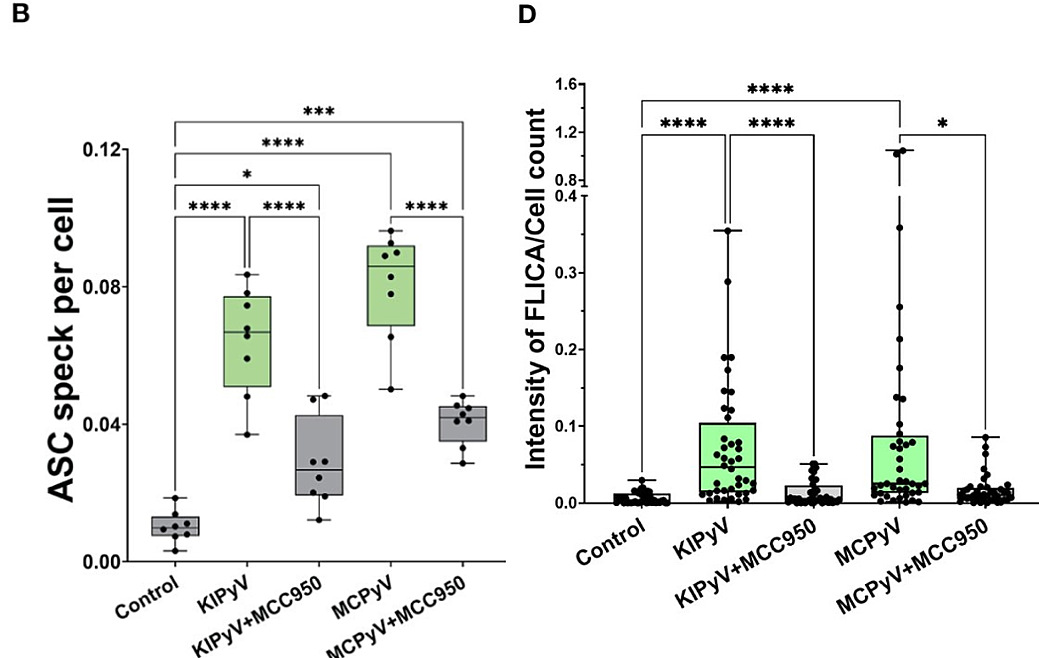
To determine whether spherical VLPs, which induced an inflammatory response in THP-1 macrophages, activated the NLRP3 inflammasome, THP-1 cells were pretreated with a small-molecule inhibitor of NLRP3 (MCC950) before exposure to VLPs. This significantly reduced the percentage of dead cells (p < 0.01) and the release of inflammasome-specific cytokine IL-1β (p<0.05 for heterogeneous VLPs 20-60 nm in size, or p<0.0001 for homogenous large 45-50 nm VLPs). In addition, ASC specks, which assemble after inflammasome activation, were detected in THP-1-ASC speck reporter cells after treatment with VLPs. Finally, active caspase-1, a component of the inflammasome signaling cascade, was detected via Western Blot in VLP-exposed macrophages and decreased when macrophages were pretreated with MCC950. Using the FLICA assay, active caspase-1 was determined to colocalize with dead cells, confirming pyroptosis. Taken together, these results suggest that spherical VLPs in THP-1 macrophages activate the NLRP3 inflammasome. Repeating the same experiments in primary human macrophages revealed a similar activation pattern.
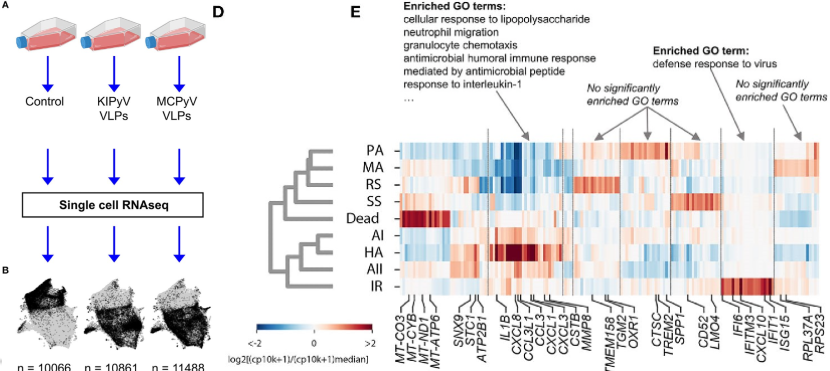
In both THP-1 macrophages and primary human macrophages, the caspase-1 and ASC speck assays revealed that the inflammasome was not consistently activated in 100% of cells; rather, only a portion had been activated by VLPs, and the percentage of pyroptic cells never exceeded 20%. So, to explore the heterogeneity of macrophage activation, single-cell RNA sequencing (scRNAseq) was performed using a modified inDrops method, where single-cell transcriptomes were barcoded in droplets using an Onyx microfluidic device. In general, gene expression patterns showed an enrichment of genes associated with immune cell activation in the THP-1 cells treated with VLPs compared to controls; gene ontology enrichment analysis identified neutrophil migration, granulocyte chemotaxis, cellular response to TNF, response to IL-1 and lymphocyte migration as highly enriched relative to controls. However, within the population of THP-1 macrophages exposed to VLPs, scRNAseq revealed multiple subpopulations that differed in gene expression and abundance, of which Lučiūnaité et al detail nine. Hierarchical clustering separated these subpopulations into two groups. One cluster was comprised of 4 subpopulations of macrophages distinguished by their inflammation-related gene expression profiles. These included “high activation”, which showed a strong inflammatory response, “activation I” and “activation II”, which had enriched inflammation-related genes but at lower levels, and “interferon response”, which are genes induced by interferon and related to a cell’s response during viral infections. The other cluster was comprised of 4 macrophage populations that did not show enrichment of inflammation-related genes (“steady state”, “resting state”, “metabolically active”, and “prone to activation”) along with dead cells in a more distant branch. This reveals that the THP-1 macrophage culture was not homogeneous in its response to exposure to VLPs. As the mechanisms resulting in differential macrophage activation are currently unknown, future studies should investigate why only some cells activate an inflammatory response when exposed to VLPs.
- Lučiūnaitė A, Dalgėdienė I, Žilionis R, Mašalaitė K, Norkienė M, Šinkūnas A, Gedvilaitė A, Kučinskaitė-Kodzė I, Žvirblienė A.
- Activation of NLRP3 Inflammasome by Virus-Like Particles of Human Polyomaviruses in Macrophages. Front Immunol. 2022 Mar 9;13:831815. doi: 10.3389/fimmu.2022.831815. PMID: 35355981; PMCID: PMC8959312.


