Riboflavin (vitamin B2) is an essential nutrient for human health. While humans cannot produce riboflavin, it is in food and is produced by microbes via fermentation. Thus, industrial production of riboflavin through Bacillus subtilis fermentation has been previously established. Studies have explored mutation engineering techniques, such as heavy ion radiation and atmospheric pressure room temperature plasma (ARTP), to enhance riboflavin production in B. subtilis. However, high-throughput screening methods for targeted strains have been underutilized, despite their potential to improve selection efficiency. Droplet microfluidics technology is a promising approach for high-throughput screening. The use of dispersed droplets allows for encapsulation of single cells, making it suitable for screening riboflavin-producing B. subtilis strains. In this study, Xu et al. prepared a library of mutants from the parent strain S1, a strain already capable of producing riboflavin due to a random mutation in BS168DR. By using droplet microfluidics, the authors identified strain U3 as having enhanced riboflavin production. Fed-batch fermentation revealed that strain U3 had an 18% increase in riboflavin yield compared to the parent strain S1. Through whole genome sequencing comparison, two specific mutation sites in U3 were identified as sinRG89R and icdD28E. These results highlight the potential of droplet microfluidics in expediting the screening process for bacterial mutants that exhibit enhanced production of targeted enzymes or molecules.
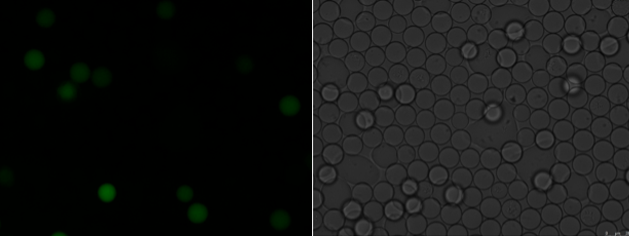
The authors generated a mutant library from the parent strain B. subtilis S1 using ARTP. The mutants were collected and encapsulated within water-in-oil droplets with a diameter of about 25 microns. Droplets containing single mutants were then incubated for an additional four hours at 37°C, when fluorescence was detected as a proxy for riboflavin production. A total of 15,000 droplets were screened at a flow rate of 400 microliter/hour. To identify a subset of mutants for further testing, the authors selected the first 2% of droplets that passed the required fluorescence intensity. This resulted in 8 mutants called U1-U8. Xu et al. grew each of these mutant strains on agar plates and noted that U3 had a notable change in colony morphology compared to the parent strain S1. U3 was denser in growth, highlighting its potential for U3 improved biomass.

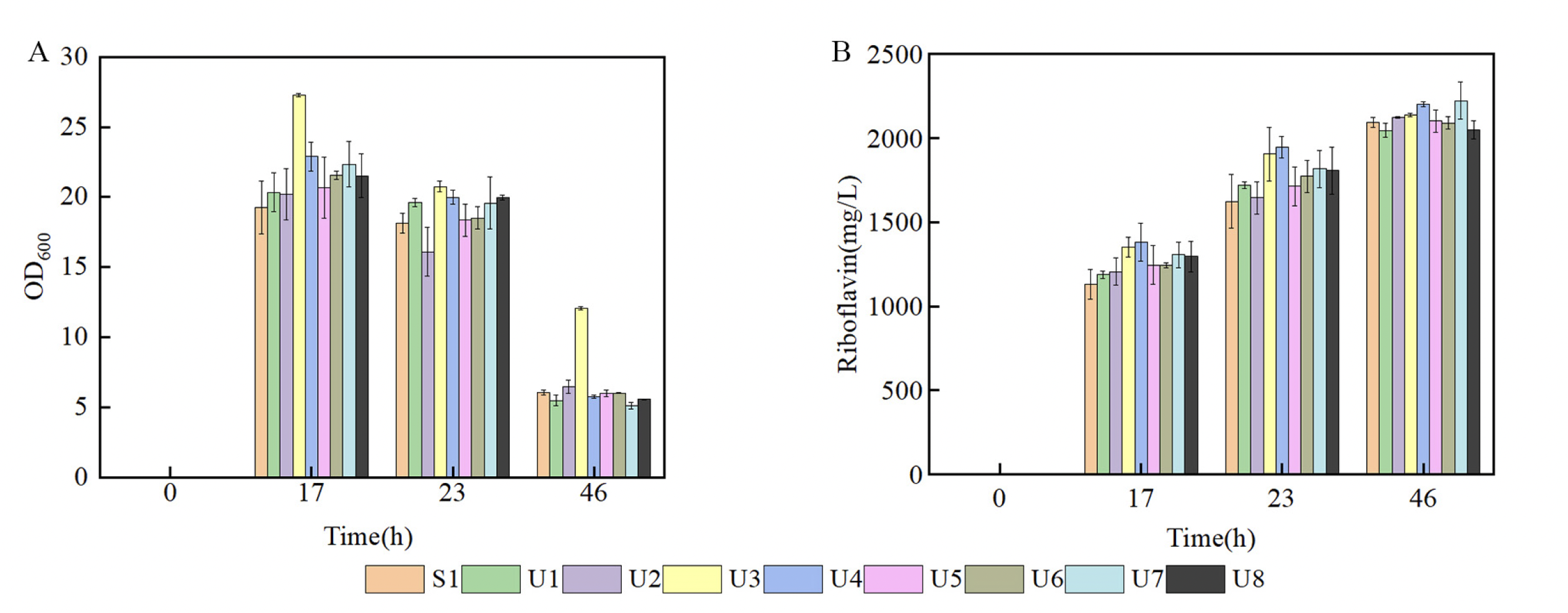
To examine the production capability of the eight different mutants identified by droplet screening, the authors grew each strain in flask with glucose and sucrose for fermentation. As a control, the authors also measured optical density of the culture as a proxy for growth. OD600 results suggested that U3 grew much faster than the parent strain S1 and the other seven mutants. Riboflavin production by U3 at 46 hours was 2138.32 mg/L, slightly higher than the control strain's production of 2093.46 mg/L. To further evaluate U3's riboflavin production ability, U3 and S1 strains were subjected to fed-batch fermentation in a 7.5-L bioreactor. Results demonstrated that U3’s riboflavin production surpassed that of S1 at 42 hours and continued to incline, with an overall yield that was 18% higher than the parent strain S1. Overall, these findings suggest that U3 demonstrated a higher demand for residual glucose and an enhanced capacity for riboflavin production compared to S1.
To investigate the genetic factors contributing to this enhanced production of riboflavin, whole genome sequencing was conducted on U3. Sequencing results revealed that U3 had two mutations in two genes, sinR and icd. These two genes are involved in biofilm synthesis and TCA cycle, respectively. In order to verify the impacts of these genes on riboflavin yield, the authors made mutations in sinR and icd in the parent strain S1 via double exchange homologous recombination. A mutation in the sinR gene resulted in remarkable growth improvement, with a substantial 60% increase observed after 24 hours and a 500% increase after 36 hours compared to the parent strain S1. However, the impact on riboflavin yield was more modest, showing only minor changes in production. In contrast, a mutation in icd had smaller impacts on growth but significantly impacted riboflavin production. In conclusion, the findings of this study highlight the potential of droplet-based microfluidics in accelerating the screening process for bacterial mutants with enhanced riboflavin production. The identification of the U3 strain, with markedly enhanced growth and notable increases in riboflavin yield, demonstrates the power of targeted genetic mutations in driving bioprocess optimization. These results demonstrate the utility of droplet screening, providing valuable insights for further optimization and genetic engineering efforts in improving industrial riboflavin production.
- Xu F, Liu C, Xia M, Li S, Tu R, Wang S, Jin H, Zhang D. Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening. Microorganisms. 2023 Apr 20;11(4):1070. doi: 10.3390/microorganisms11041070. PMID: 37110496; PMCID: PMC10146818.


