As environmental pollution continues to threaten the quality of available water resources around the globe, deploying technologies such as nano-adsorbants for water treatment is becoming a critical component of responsible and sustainable water management. Chitosan is a widely available, non-toxic, and biodegradable polysaccharide with biosorbant properties that make it an excellent candidate for water treatment. However, directly applying chitosan powder to polluted water would be impractical, as it would be difficult to separate the chitosan from the feed water following adsorption and could thus act as a secondary pollutant. As a result, significant research efforts have been devoted to the fabrication of modified chitosan structures, such as beads, hydrogels, and films, that could be applied as an adsorbent and easily removed from the feed water. In this study, Zhang et al from Xiamen University used co-flow droplet microfluidics to generate even-sized chitosan microspheres with magnetic properties. The spherical shape and magnetic properties would allow these chitosan microspheres to be easily separated from water by a magnetic field.
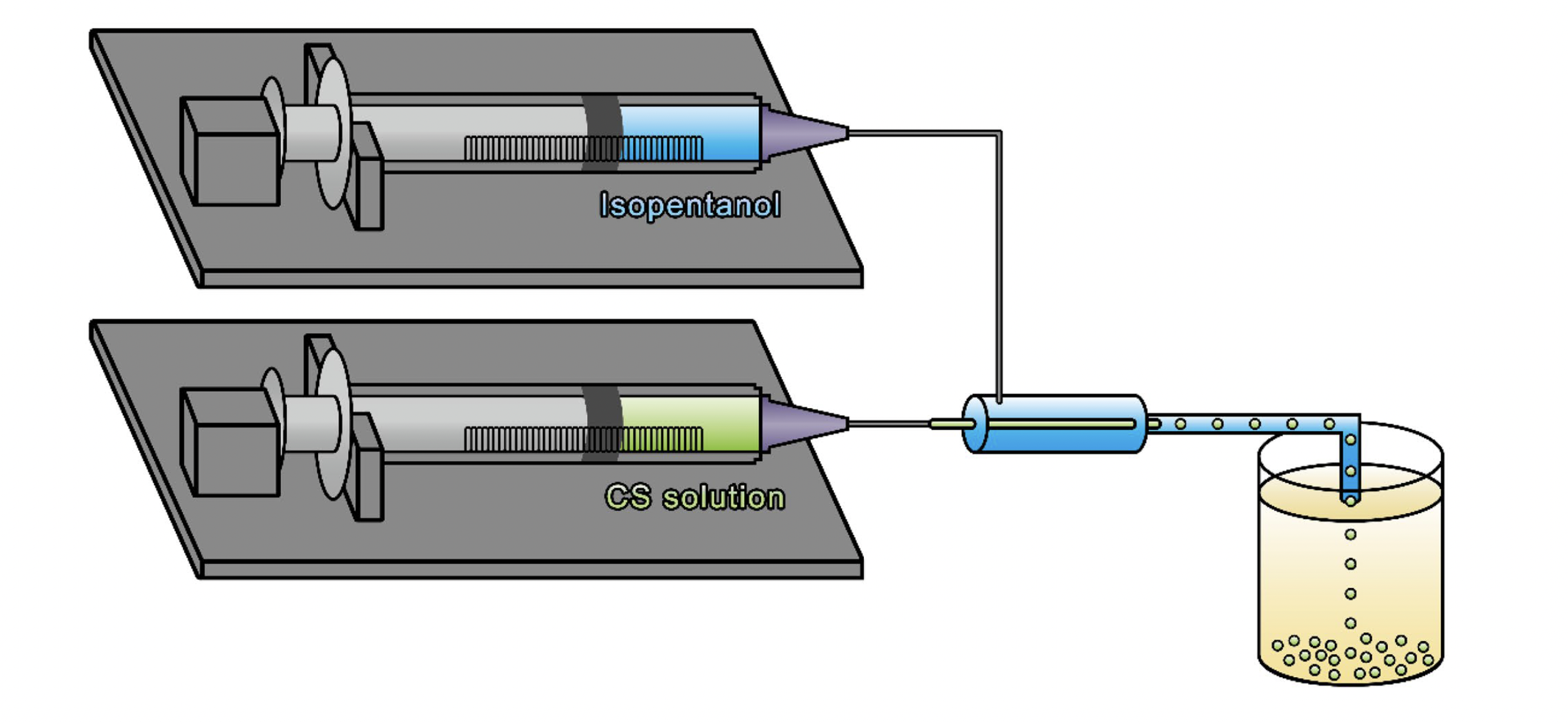
To prepare the magnetic chitosan solution for injection into the co-flow droplet microfluidic device, iron (II) chloride tetrahydrate and iron (III) chloride hexahydrate were co-dissolved in water while chitosan powder was dissolved in 5 wt% acetic acid solution. The iron solution and chitosan solution were then mixed and added to the microfluidic device. Isopentanol was used as the continuous phase. In co-flow droplet microfluidics, the aqueous phase is cut into uniform droplets by the shear force of a fast-flowing, immiscible continuous phase. Here, isopentanol was chosen as the continuous phase because it is partially miscible with water, so that once the aqueous chitosan solution was cut into droplets, some of the water in the droplets could be absorbed into the isopentanol phase, leading to solidification and helping to maintain a spherical shape. As a result, no surfactant nor cross-linking agent were required to stabilize the chitosan droplets. The pre-solidified magnetic chitosan droplets were carried to a 25% ammonia aqeuous solution and stirred at room temperature for 4 hours. The solidified microspheres were collected, washed with water, and dried at 60°C.
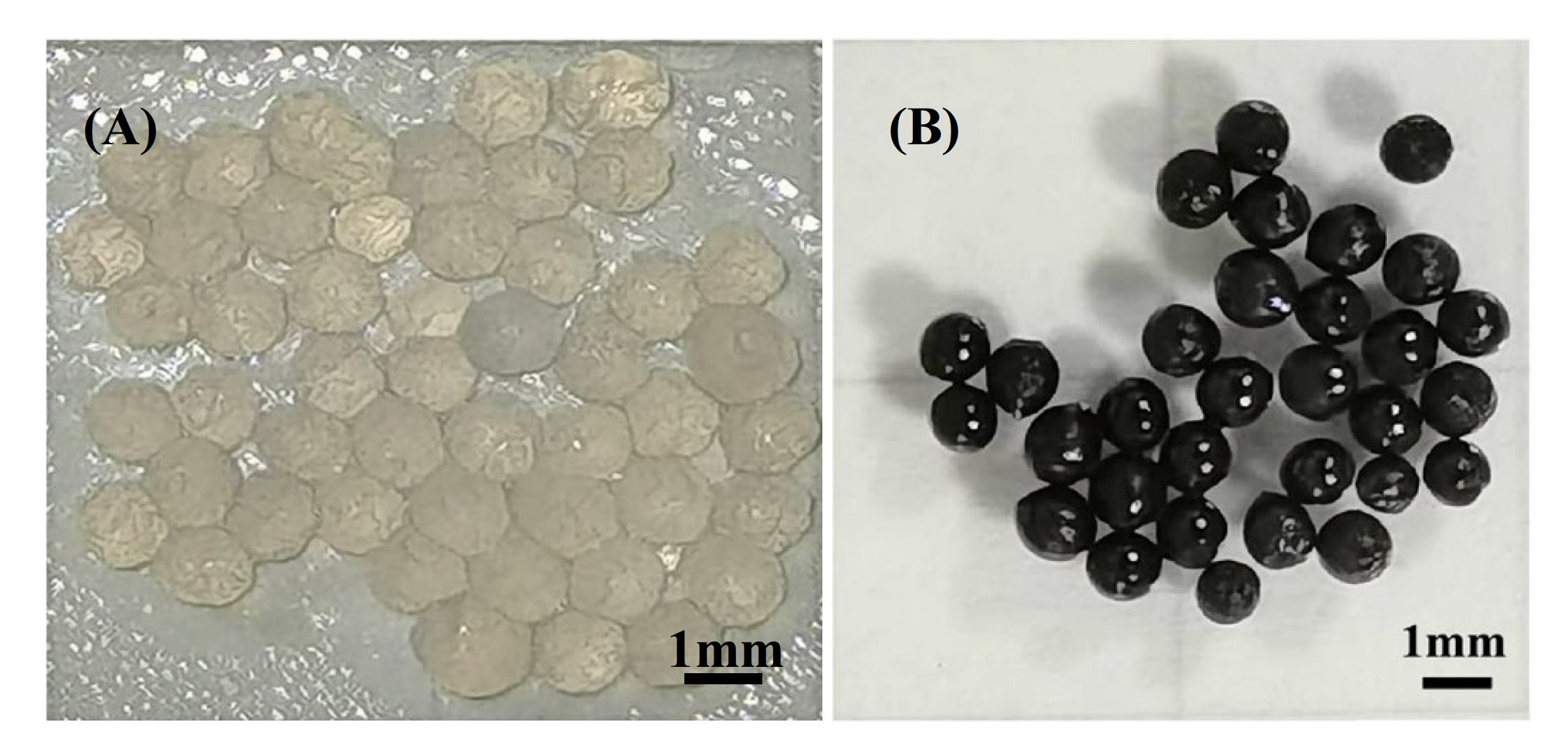
After drying, the resulting magnetic chitosan microspheres measured 406 ± 9µm, displaying the uniform size distribution that is a product of droplet microfluidic generation. The microspheres had a spherical/ellipsoid shape and a smooth surface with a few wrinkles, like a walnut. Fourier transform infrared (FT-IR) characterization revealed both the typical bands of the chitosan polysaccharide but also a strong band at 594 cm-1, which corresponds to the magnetite Fe3O4. This suggests that the co-precipitated Fe2+ and Fe3+ form in situ Fe3O4 nanoparticles via acid-base neutralization in the ammonia solution. Transmission electron microscopy (TEM) imaging revealed that the formed Fe3O4 nanoparticles are evenly dispersed in and firmly stabilized by the chitosan matrix. Analysis also illustrated the superparamagnetic property of the microspheres, which would allow them to be easily removed from water following adsorption.

Lastly, to demonstrate the adsorption capacity of the magnetic chitosan microspheres, 150 mg chitosan microspheres were added to a 250 mg/L Congo red solution and lighted stirred at room temperature. The supernatant was sampled at various time points and the concentration of Congo red was determined at 498 nm. Compared to the chitosan microspheres without iron, the magnetic chitosan microspheres had a higher adsorption capacity within 60 minutes. As a result, the magnetic chitosan microspheres produced by co-flow droplet microfluidics are excellent adsorbant candidates for water treatment.


