Plasma cells (PCs) play a crucial role in humoral immunity by continuously producing and secreting antibodies. However, the mechanisms governing their secretory function and survival remain poorly understood. This knowledge gap hampers the development of effective therapies targeting PCs. In this study, the authors identified a protein called Sec22b, a member of the SNARE family, as an important regulator in PCs. SNARE proteins are involved in intracellular transport and fusion of protein cargoes between organelles. Through experiments using a mouse model and droplet microfluidics, the authors found that Sec22b is essential for PC maintenance and a robust immune response following vaccination or infection. Mechanistically, Sec22b controls the expression of genes involved in cell cycle, mitochondrial function, and endoplasmic reticulum (ER) structure. These findings shed light on the regulation of organelle homeostasis in PCs and highlight the critical role of Sec22b in normal humoral responses.
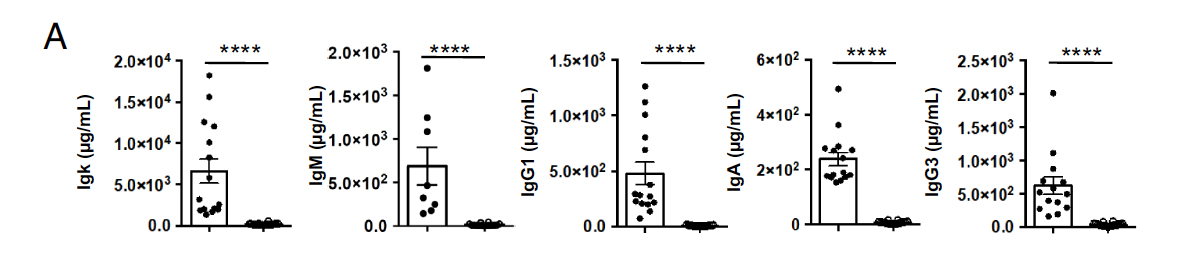
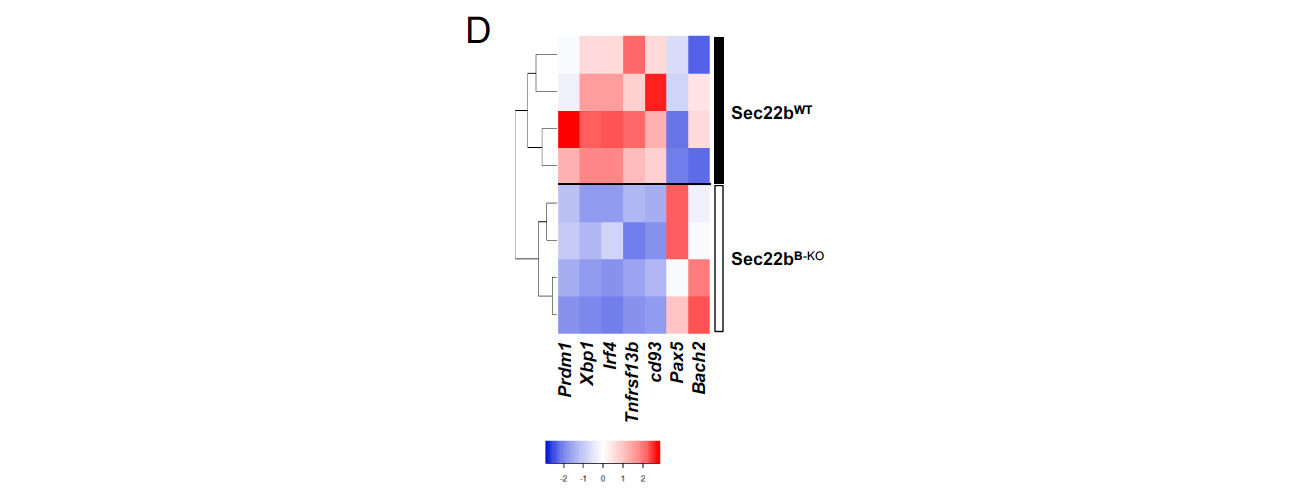
To investigate the impact of Sec22b on PC maintenance and antibody secretion, the authors conducted a study using a mouse model in which Sec22b expression was specifically eliminated in B cells (referred to as Sec22bB-KO). Analysis of RNA-sequencing results indicated that Sec22b is consistently expressed at low levels during B cell differentiation. The authors also found that Sec22b deficiency did not significantly affect B cell development, except for a slight reduction in mature B cells in the bone marrow and spleen. However, Sec22bB-KO mice exhibited a severe deficiency in circulating antibodies across all tested isotypes. Specifically, the levels of Igκ, which represents the majority of secreted antibodies, were reduced by more than 40-fold. IgG1 titers were 55 times lower, while IgM, IgA, and IgG3 were reduced by 40, 30, and 20 times, respectively. This antibody deficiency was associated with a 10-fold decrease in the frequency and absolute number of plasma cells in the spleen and bone marrow, particularly affecting mature CD19−B220− plasma cells. Furthermore, the remaining splenic plasma cells in Sec22bB-KO mice exhibited a less mature profile, with decreased expression of plasma cell markers and master regulators, such as Cd93, Tnfrsf13b, Prdm1, Xbp1, and Irf4, while showing enhanced expression of B cell master regulators Pax5 and Bach2 at the transcriptional level. These findings highlight the crucial and distinct role of Sec22b in the maintenance of plasma cells and the regulation of circulating antibody levels under steady-state conditions.
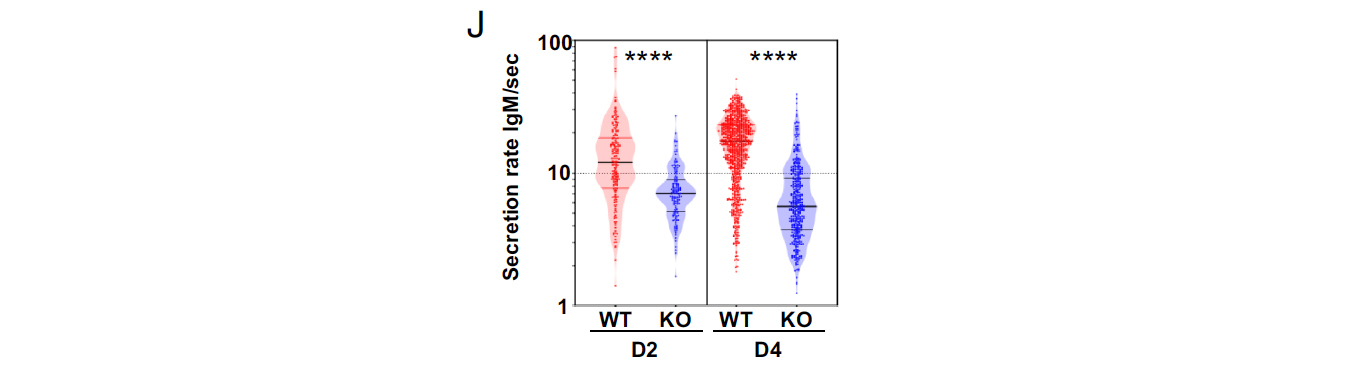
To investigate the role of Sec22b in PC differentiation and antibody secretion, in vitro differentiation assays were performed using splenic B cells. The results showed that while PC frequency doubled between day 2 and day 4 in control cultures, it remained constant in Sec22bB-KO cultures. This was accompanied by increased apoptosis and impaired cell cycle progression in Sec22b-deficient PCs, particularly evident on day 4. These findings indicate that Sec22b is not necessary for the initiation of PC differentiation but is crucial for the maintenance and expansion of this cell subset. To determine if Sec22b directly regulates antibody secretion, a droplet microfluidic-based technique was employed to assess secretion rates at the single-cell level. The results showed that Sec22b-deficient PCs had a reduced secretion rate of IgM compared to WT PCs. On average, Sec22b-deficient PCs secreted 2 to 4 times less IgM/s compared to WT PCs on both day 2 and day 4 of in vitro culture. This indicates that Sec22b deficiency leads to a significant decrease in antibody secretion, contributing to the marked reduction in serum antibody levels observed in Sec22b-deficient mice. Overall, the findings suggest that Sec22b is not only essential for the maintenance and expansion of PCs but also plays a role in regulating antibody secretion, likely through its influence on transcriptional programs and cargo transport mechanisms.
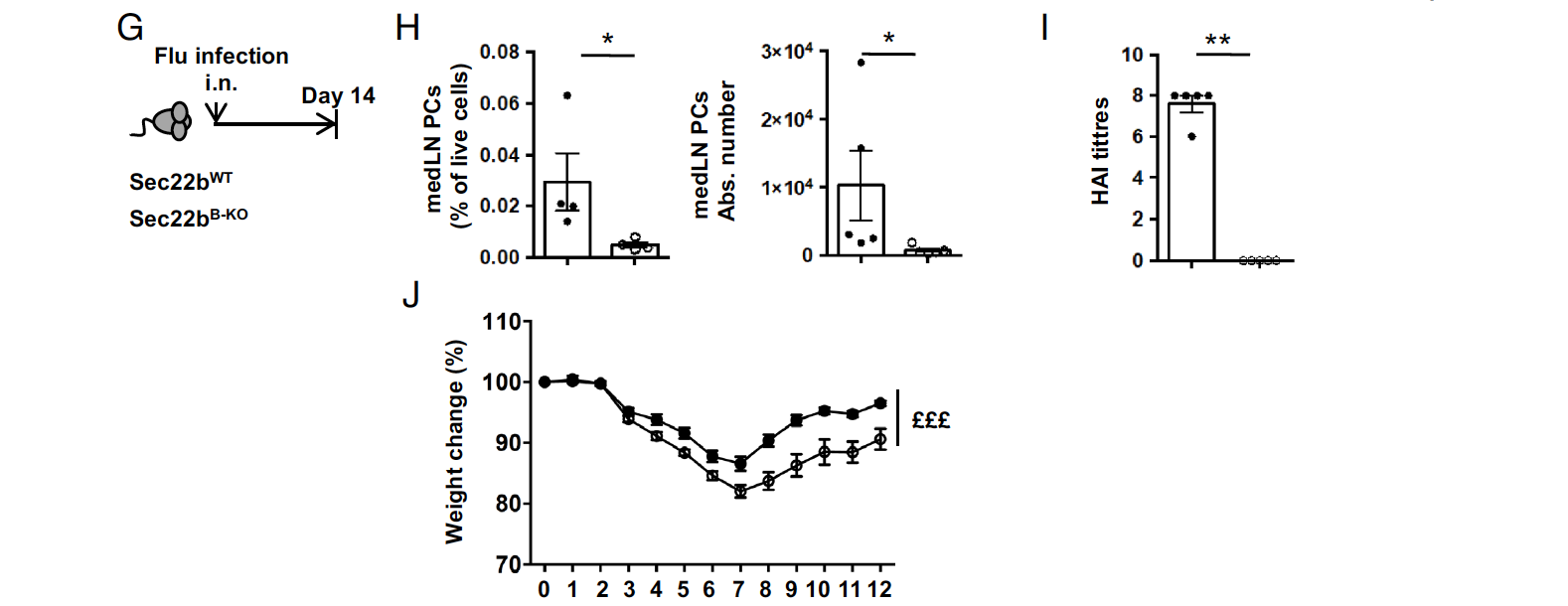
To further evaluate the impact of Sec22b deficiency on PCs and the production of circulating antibodies, the researchers conducted various immunization and infection experiments. First, after T-dependent immunization with sheep red blood cells (SRBCs), Sec22bB-KO mice exhibited an extremely low frequency and number of PCs in the spleen, reduced by over 100 times compared to controls. Correspondingly, antibody titers in response to SRBC immunization were significantly decreased in Sec22bB-KO mice. During influenza A infection, Sec22b-deficient mice displayed a profound defect in the frequency and number of PCs in the draining mediastinal lymph nodes. Additionally, flu-specific antibodies were undetectable in the serum of Sec22b-deficient mice. These deficiencies were associated with exacerbated weight loss in Sec22bB-KO mice, indicating more severe infections in the absence of Sec22b. These findings demonstrate that Sec22b is essential and plays a nonredundant role in establishing a potent and efficient humoral immune response following both vaccination and infection scenarios. Sec22b deficiency resulted in significantly reduced PC frequencies, impaired antibody production, and compromised control of viral infection. Moreover, the authors utilized droplet microfluidics as a technique to detect antibody secretion, showcasing its valuable application in the field of antibody research.
- Bonaud A, Gargowitsch L, Gilbert SM, Rajan E, Canales-Herrerias P, Stockholm D, Rahman NF, Collins MO, Taskiran H, Hill DL, Alloatti A, Alouche N, Balor S, Soldan V, Gillet D, Barbier J, Bachelerie F, Smith KGC, Jellusova J, Bruhns P, Amigorena S, Balabanian K, Linterman MA, Peden AA, Espéli M.
- Sec22b is a critical and nonredundant regulator of plasma cell maintenance. Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2213056120. doi: 10.1073/pnas.2213056120. Epub 2023 Jan 3. PMID: 36595686; PMCID: PMC9926242.


