Spheroids are three-dimensional aggregates of cells that more accurately reflect the cell morphology, viability, proliferation, stimuli response, drug metabolism, and gene expression of in vivo tissue than conventional two-dimensional cell cultures. As spheroids retain intercellular and cell-extracellular matrix interactions, they hold immense potential for cancer biology research and pharmaceutical screening campaigns. Spheroid engineering within microfluidic droplets promotes the production of homogenous spheroids with high throughput and precision. However, a key limitation of traditional water-in-oil droplets is their impermeable nature, which hinders the exchange of liquid media. This results in restricted nutrient availability for enclosed cells and an inability to remove cell metabolites. Semi-permeable capsules (SPCs) are a breakthrough microfluidic technology that offer the same single-cell encapsulation and high-throughput capabilities of traditional droplets but are surrounded by a porous hydrogel shell that enables the exchange of liquid media. When suspended in a growth medium, SPCs facilitate unrestricted diffusion of essential nutrients and metabolites while securely encapsulating the proliferating cells. As a result, SPCs are better suited to support spheroid formation. Here, both suspension K-562 cells and adherent CHO-K1 cells are used to form spheroids in SPCs.
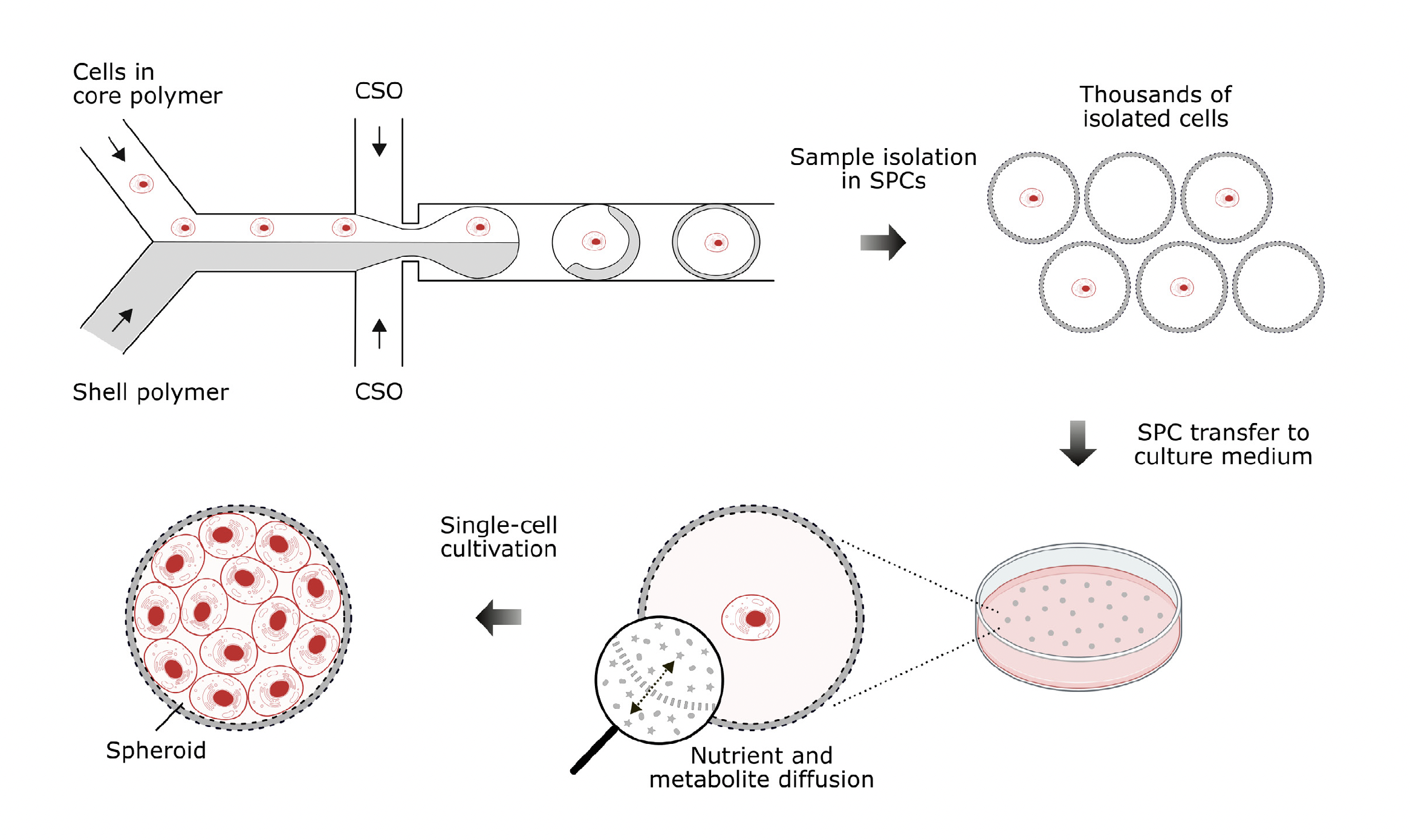
The K-562 cells were cultivated in RPMI medium with 10% fetal bovine serum and 1x penicillin-steptomycin at 37°C in 5% CO2. Cells were stained with Trypan Blue dye to assess cell viability. The final concentration of viable cells was 7.7M/ml, corresponding to ~20% of SPCs containing at least one cell. The CHO-K1 cells were cultured in F-12 medium with 10% fetal bovine serum and 1x penicillin-streptomycin at 37°C in 5% CO2. After reaching ~70-80% confluency, the cells were passaged and then stained with Trypan Blue dye to assess cell viability. The final concentration of viable cells was ~3.8M/ml, ensuring that approximately 10% of SPCs contain at least one cell; the lower SPC occupancy was chosen to avoid cell clumping. Both cell suspensions were encapsulated in SPCs using the SPC Innovator Kit and the Onyx system for SPC generation. The collected SPCs were transferred to Petri dishes prefilled with 4 ml of complete culture medium and cultivated at 37°C in 5% CO2.
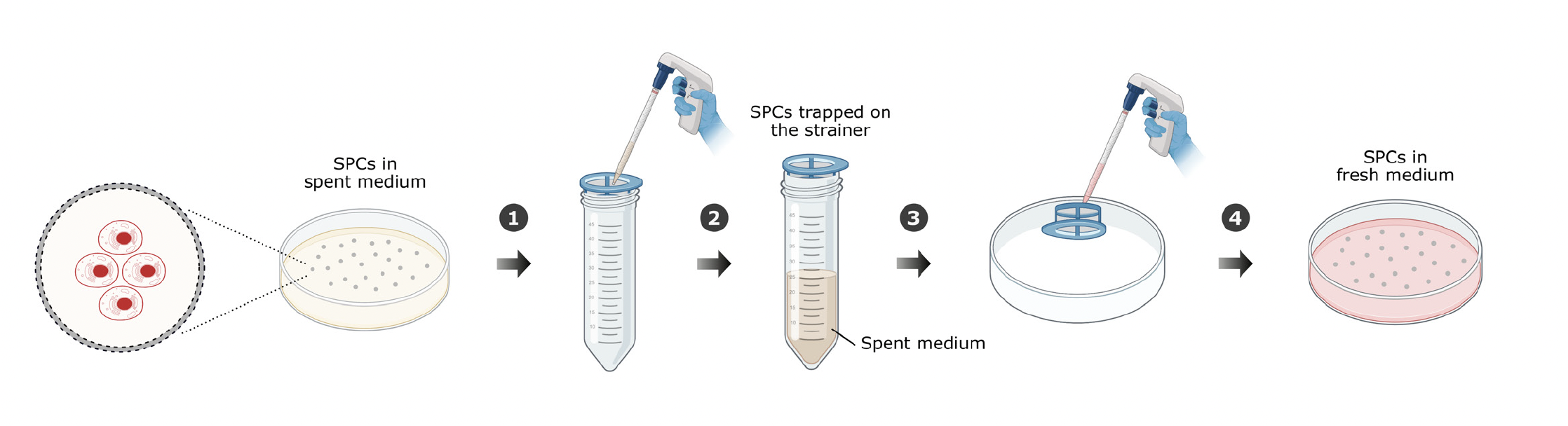
After 2 hours in culture, 500 µL of SPCs were removed from each Petri dish, stained with SYTO 9 and ethidium homodimer-1, incubated for 30 minutes, and imaged to assess viability. For the K-562 cells, viability before encapsulation in SPCs was 99.7%; after encapsulation in SPCs, viability was 76.7%. For the CHO-K1 cells, viability before encapsulation in SPCs was 98.4%; after encapsulation in SPCs, viability was 86.7%. The single-cell cultures in SPCs were allowed to grow until the core of the SPCs was filled with cells. Every 3-4 days, the culture medium was changed using the cell passaging workflow depicted in Figure 2. This ensured that the growing cell culture had access to a continuous supply of nutrients and that cell metabolites could be removed from the SPCs.
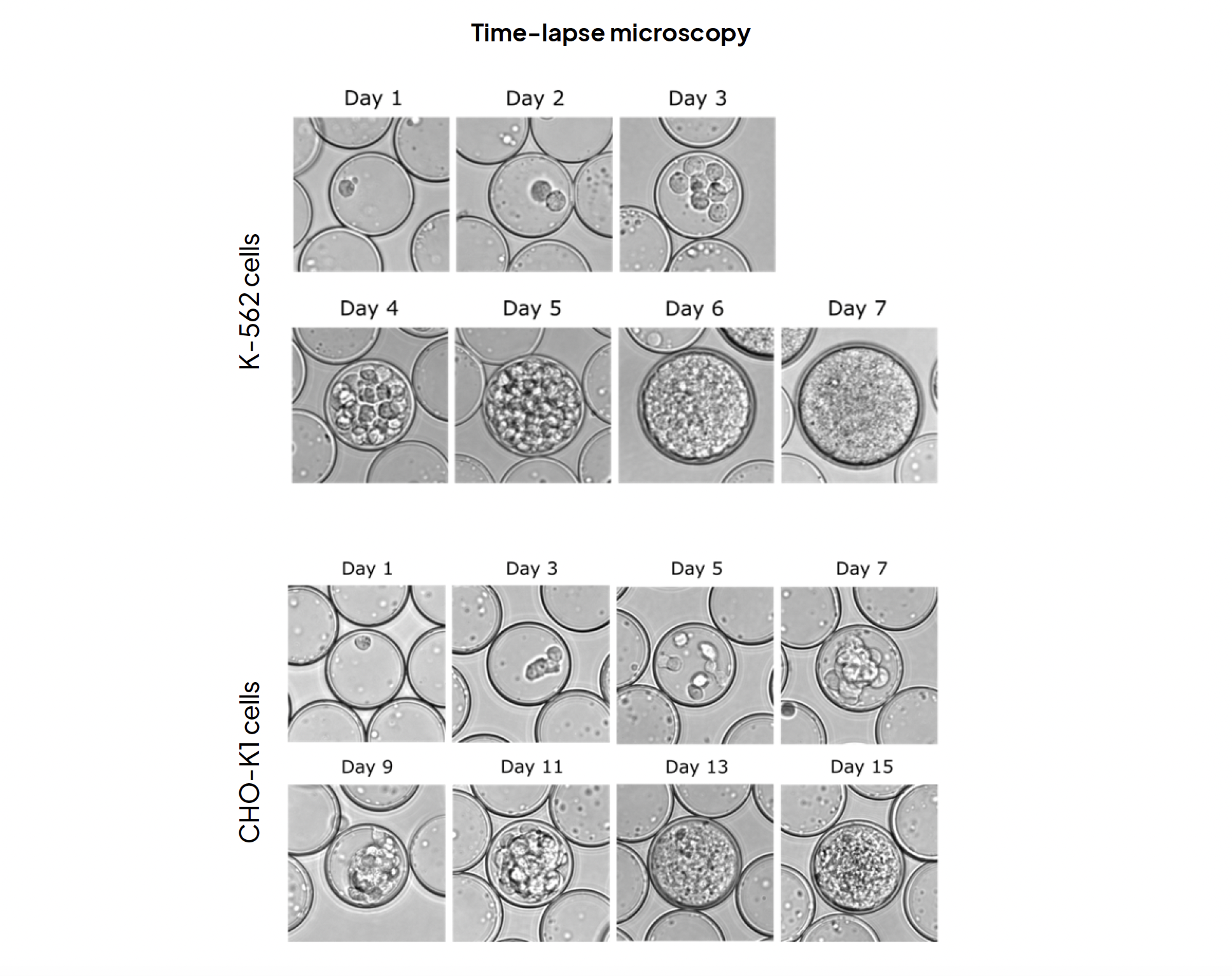
Single isolated K-562 cells formed spheroids in approximately 7 days, with live-dead staining conducted on day 7 indicating a spheroid formation efficiency of approximately 80%. Single isolated CHO-K1 cells formed spheroids in approximately 15 days, with live-dead staining on day 15 revealing a spheroid formation efficiency of around 60%. Throughout these experiments, semi-permeable capsules facilitated the isolation of individual viable mammalian cells, maintained stability throughout the cultivation period, and actively promoted cell proliferation by allowing liquid media exchange, ultimately leading to the formation of minispheroids. These combined factors highlight the potential of SPC technology to drive advancements in 3D cell culture research.
- Application Note Download
- Summary prepared by Andréa Covey for Atrandi Biosciences


