Amid the global challenge of plastic waste, there is a growing urgency to explore eco-friendly solutions that address the accumulation and incineration of non-biodegradable polymers. Shifting toward polyester-based plastics and the large-scale cultivation of microorganisms capable of breaking down polyester presents a promising alternative to conventional plastics. Limited documentation of polyester-depolymerizing enzymes to date underscores the inadequacies of the current plate-based screening method, which is time-consuming, susceptible to false positives, and has likely impeded the comprehensive investigation of these enzymes. Droplet microfluidic-based fluorescence-activated droplet sorting (FADS) has emerged as a powerful tool for high-throughput screening in enzyme evolution, single-cell analysis, and enhancing microbial production of extracellular compounds. Yet, the absence of suitable fluorescent probes for polyester degradation has hindered the utilization of FADS in this context. In this study, researchers from Nanjing Tech University developed a synthetic fluorescent polyurethane analogue probe (FPAP), enabling high-throughput FADS screening for polyester-degrading enzymes.
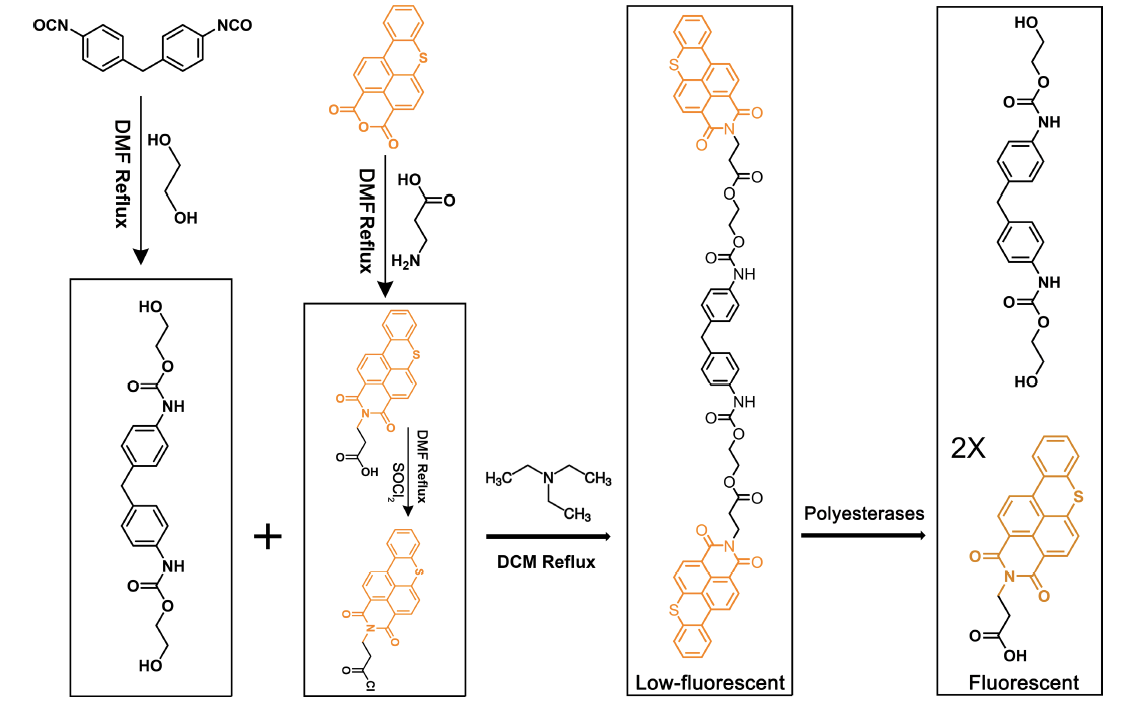
The synthetic probe, FPAP, was designed by structurally mimicking polyurethane (PUR) monomers. This is a significant advancement, as the only other existing probe for screening polyester-degrading enzymes, FDBz, is specific for PET-polyesterases, while there is currently no reported probe for the detection of PUR-polyesterases in the literature. The probe features fluorogenic molecules tagged to both sides of the PUR monomer so that when acted upon by polyesterases, it releases fluorescein, leading to a significant increase in fluorescence intensity. This property enables FPAP to be deployed for FADS and streamlines the screening of microorganisms for PUR-degrading enzymes. The probe was found to be non-toxic to common model microorganisms, including Escherichia coli, Pseudomonas aeruginosa and Saccharomyces cerevisiae.
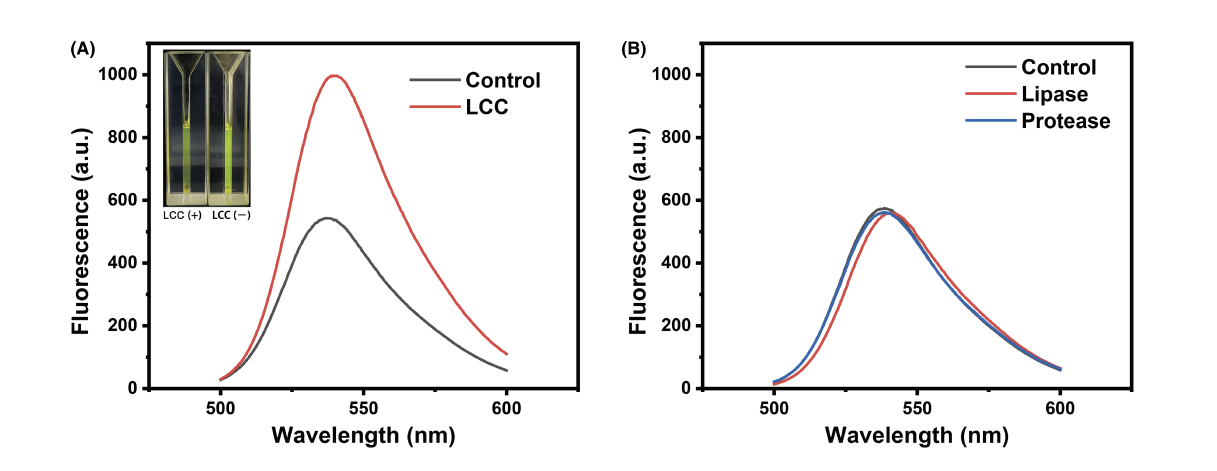
One major limitation encountered in the impranil DLN-based agar plate screening for polyester-degrading enzymes is its lack of specificity, resulting in false positives from clones expressing protease- or amidase-like enzymes alongside those with the desired polyesterase activity. As a result, a critical criterion for the deployment of FPAP in FADS was specificity in its polyesterase-targeting capability. To evaluate this specificity, FPAP's response to lipase, protease, and leaf and branch compost cutinase (LCC) was compared. LCC, a PET-polyesterase with reported PUR activity, significantly increased fluorescence, indicating FPAP's sensitivity in distinguishing polyesterases from other enzymes. Conversely, the addition of lipase and protease left FPAP's chemical structure unchanged, resulting in overlapping fluorescence curves. This underscores FPAP's potential as a powerful tool for polyesterase discrimination in FADS. It is important to note that the FPAP probe's specific targeting of PUR-polyesterases remains uncertain since enzymes exclusively dedicated to PUR depolymerization have not been reported in the existing literature. Consequently, it is not feasible to compare the activity of PET- and PUR-polyesterases using the FPAP substrate. However, given the probe was synthesized based on PUR structure, it is likely that it will select for PUR-degrading enzymes.
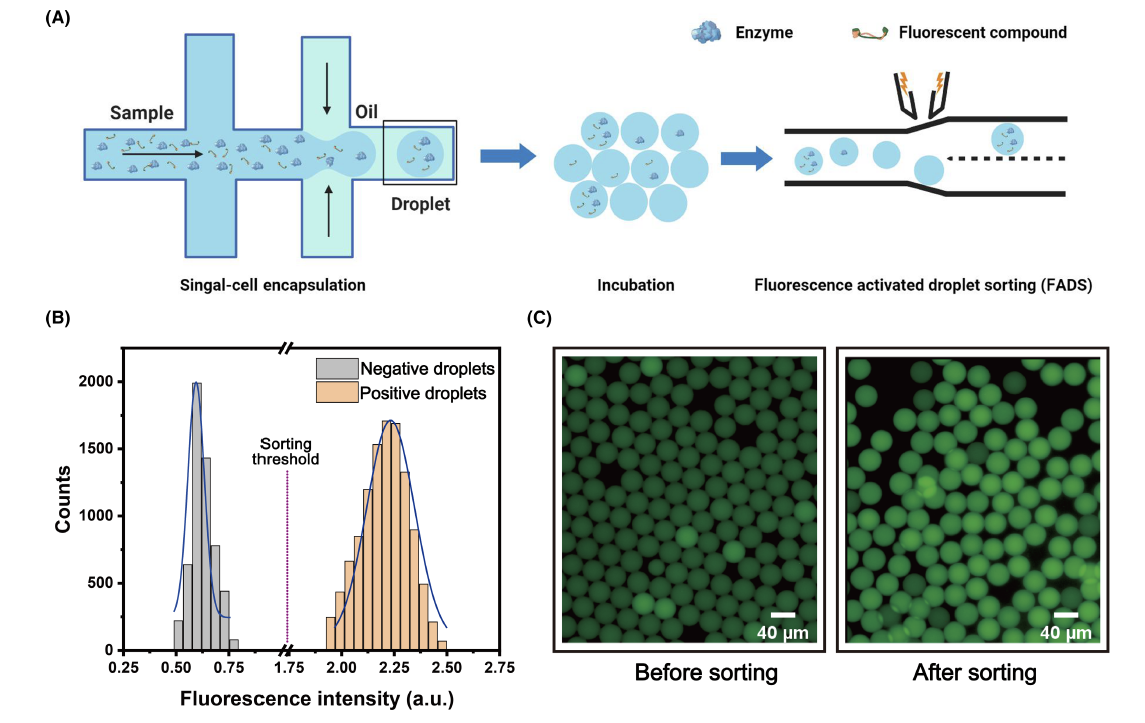
To demonstrate the utility of FPAP in a FADS screening pipeline, the probe was encapsulated within droplets in the presence of LCC enzymes to create positive droplets, while negative droplets contained FPAP alone. A mixed population of droplets was prepared comprising 50% positive and 50% negative droplets. After 6 hours of incubation, these droplets were introduced into a microfluidic device for analysis. The device continuously recorded fluorescence as each droplet passed through, revealing two distinct sub-populations with different fluorescent signals. This data was used to set a sorting threshold for FADS between positive and negative droplets. In a subsequent test, a population of droplets containing 5% positive droplets was prepared and injected into the microfluidic device for FADS. Droplets with higher fluorescent signals were directed into a collection channel during the sorting process. After sorting, droplets exhibiting higher fluorescence accounted for 95% of the total sorted droplets. These findings underscore the FPAP fluorescent probe's potential, with a droplet microfluidic system, for screening polyester-degrading microorganisms and their associated enzymes.
- Xu A, Liu J, Cao S, Xu B, Guo C, Yu Z, Chen X, Zhou J, Dong W, Jiang M. Application of a novel fluorogenic polyurethane analogue probe in polyester-degrading microorganisms screening by microfluidic droplet. Microb Biotechnol. 2023 Feb;16(2):474-480. doi: 10.1111/1751-7915.14121. Epub 2022 Jul 26. PMID: 35881631; PMCID: PMC9871523.


