Radiochemotherapy (RCT) is a comprehensive and integrated treatment approach that combines the use of radiation therapy and chemotherapy to effectively target and combat cancer cells. The effects of RCT, however, are not limited to malignant cells but also immune and stromal cells in the tumor microenvironment. Previous work suggests that RCT leads to a decrease in T cell counts. Bulk RNA-sequencing of sorted T cells revealed that RCT stimulates the expression of costimulatory CD28 found on T cells and downregulated expression of checkpoint inhibitor PD-1/PD-L1 in cervical cancer. However, these results from bulk RNA-sequencing only provide an averaged expression and could not differentiate between cell-subtype-specific gene expression. Here, the authors used single-cell RNA-sequencing to define transcriptomic remodeling of a range of cell types induced by RCT in cervical cancer. From 10 paired biopsies of cervical cancer tumors, the authors found that RCT resulted in upregulation of MHC-II genes in epithelial cells. The authors also found that tumor-associated lymphocytes, NK and T cells, were profoundly affected by RCT. CD16+ NK cells highly expressed cytotoxic genes, which were associated with favorable clinical outcomes. In contrast, T cells did not exhibit cytotoxic properties. These findings highlight the intricate responses of the tumor microenvironment to RCT that could only be resolved with single-cell RNA-seq, providing evidence of innate immunity activation and upregulation of MHC class II in cervical cancer.
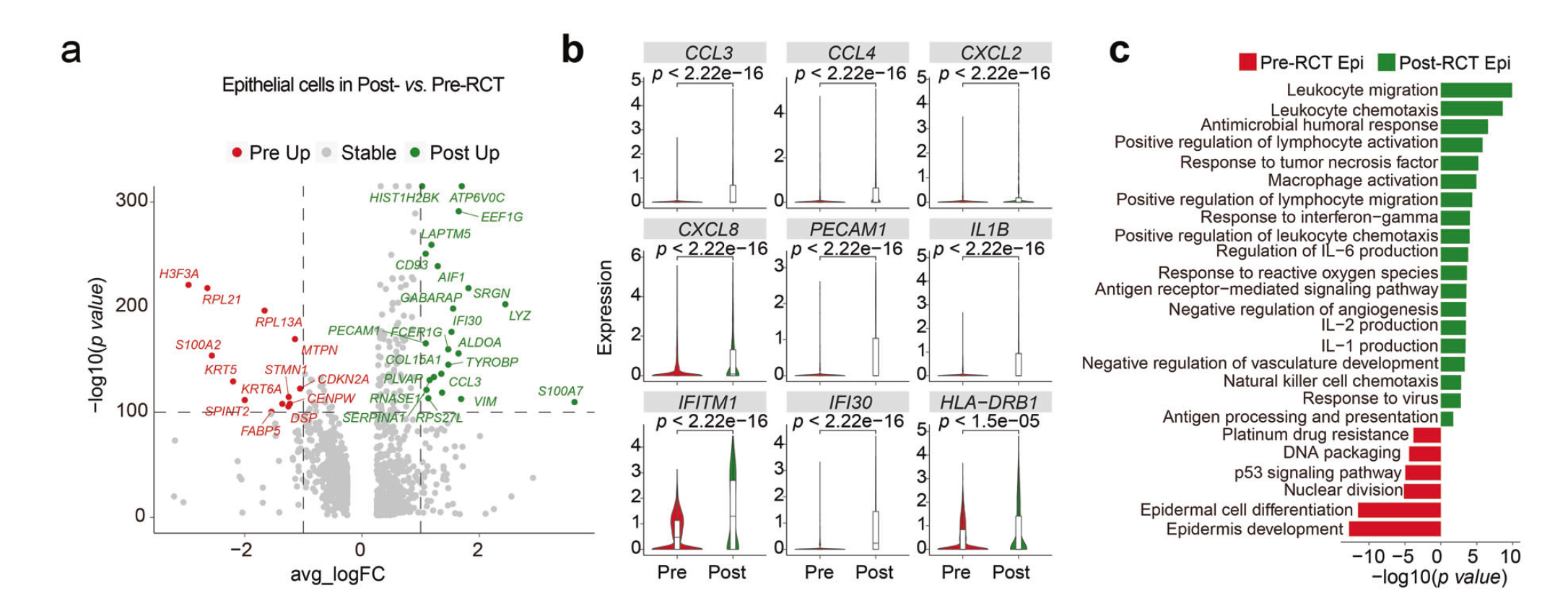
The authors collected 10 paired samples of tumor tissue from one week before RCT (pre-RCT) and three weeks following RCT (post-RCT) from five cervical cancer patients. The authors also collected tissue adjacent to tumors for comparison of tumor tissue to normal tissue (Normal). A total of nine cell clusters were identified, all of which were present in normal, pre-RCT, and post-RCT samples. However, the cellular composition was different across tissue types. Epithelial cell relative abundance was significantly reduced in all patients (P=0.0079). Five epithelial cell subclusters, labeled Epi1-5, were identified, each exhibiting distinct gene expression patterns. Epi1 displayed genes associated with tumorigenesis and immune evasion, Epi2 showed immune-related genes, and Epi5 exhibited cell cycle genes. RCT altered the proportions of these subclusters, particularly increasing Epi2 and decreasing Epi3. Results suggested that RCT had overall immunogenicity-enhancing effects, upregulating genes involved in leukocyte chemotaxis, lymphocyte activation, and antigen presentation. Notably, the expression of MHC-II, important for antigen presentation, was significantly increased in epithelial cells and Epi1/4/5 post-RCT. However, Epi2 showed a downregulation of MHC-I expression. These findings were consistent with the gene expression patterns observed in patients, although there was some heterogeneity. Immunohistochemical staining of validation samples confirmed the upregulation of MHC-II genes in epithelial cells post-RCT. In addition, RCT downregulated the expression of genes associated with malignant cervical cancer features and resulted in a reduction in tumor cell numbers overall, although a significant number of aneuploid cells remained in Epi2/3 after RCT. These cells exhibited low expression of genes involved in antigen presentation but relatively high levels of precancerous and pro-metastatic markers. Overall, RCT induced a broad immune-related response in epithelial cells, while some residual cells displayed potential malignant characteristics and reduced antigen presentation gene expression.
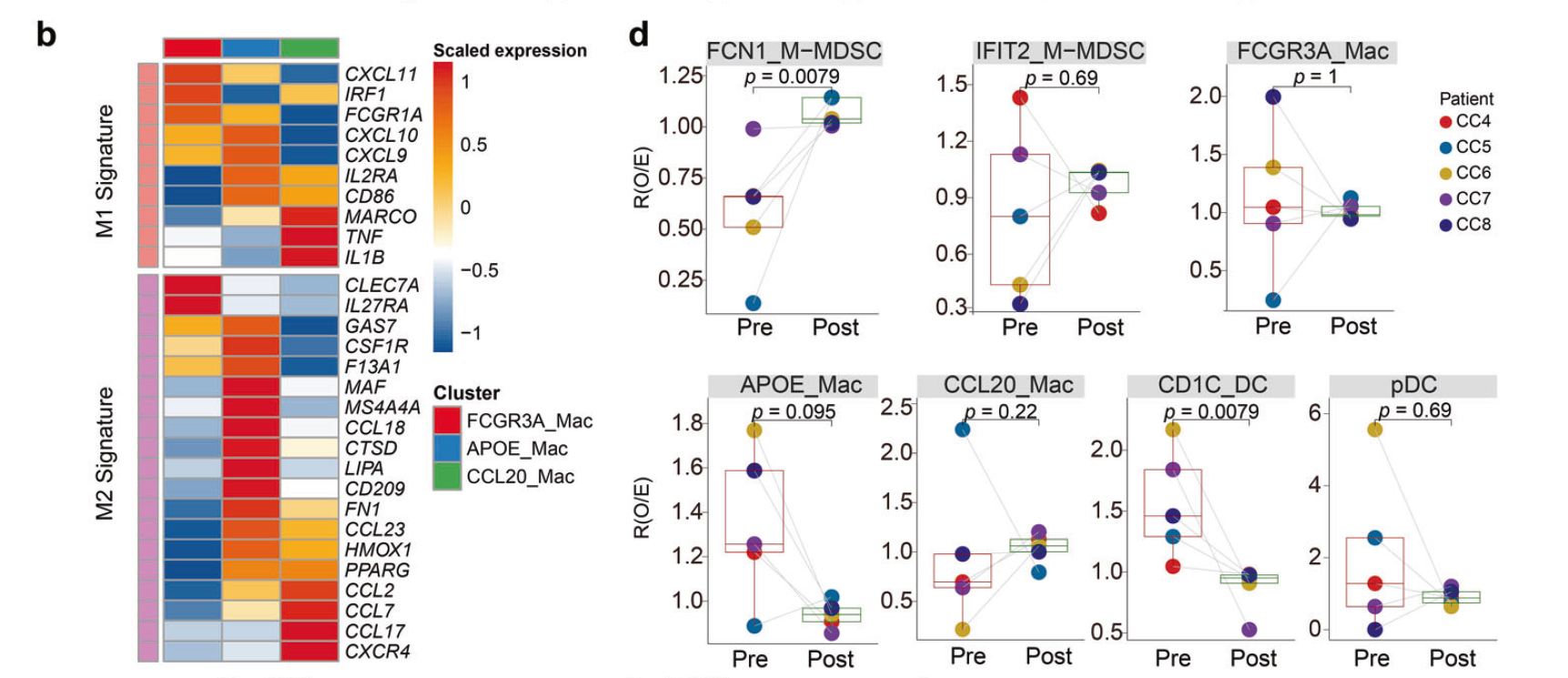
In contrast to epithelial cells, myeloid cells were elevated in post-RCT samples (P=0.0079). These trends are consistent with treatment-induced apoptosis and infiltration of myeloid cells to the tumor. The researchers turned their attention to myeloid cells, known for their impact on tumor plasticity and progression. By analyzing the expression of marker genes, seven diverse subclusters within the myeloid cell population were identified, including three subtypes of macrophages, two types of monocytic myeloid-derived suppressor cells (M-MDSCs), and two populations of dendritic cells (DCs). Two of these subclusters, APOE_Mac and CCL20_Mac, exhibited a pro-tumoral potential, with APOE_Mac expressing complement genes associated with immunosuppression and CCL20_Mac expressing genes for immunosuppressive chemokines. In contrast, the IFIT2_M-MDSC subcluster predominantly expressed pro-inflammatory genes like IFIT2 and CXCL10, which have the potential to promote anti-tumor immunity. These findings indicated the presence of both pro-tumor and anti-tumor subtypes within the myeloid cell population. To further investigate this phenomenon, the authors quantified pro-inflammatory M1-like and anti-inflammatory M2-like signatures in the myeloid cells. They observed elevated pro-inflammatory signatures in all subclusters post-RCT. RCT significantly upregulated genes involved in chemotaxis and antigen processing and presentation in myeloid cells. Gene ontology analysis confirmed an increase in genes related to leukocyte migration and activation in M-MDSCs, macrophages, and DCs post-RCT. Moreover, the enrichment of antigen processing and presentation was evident in M-MDSCs and DCs post-RCT. RCT also led to the accumulation of FCN1_M-MDSCs with upregulated pro-inflammatory features and downregulated anti-inflammatory features. Thus, this alteration of the immunological balance in cervical cancer tumors induced pro-inflammatory gene expression in myeloid cells.
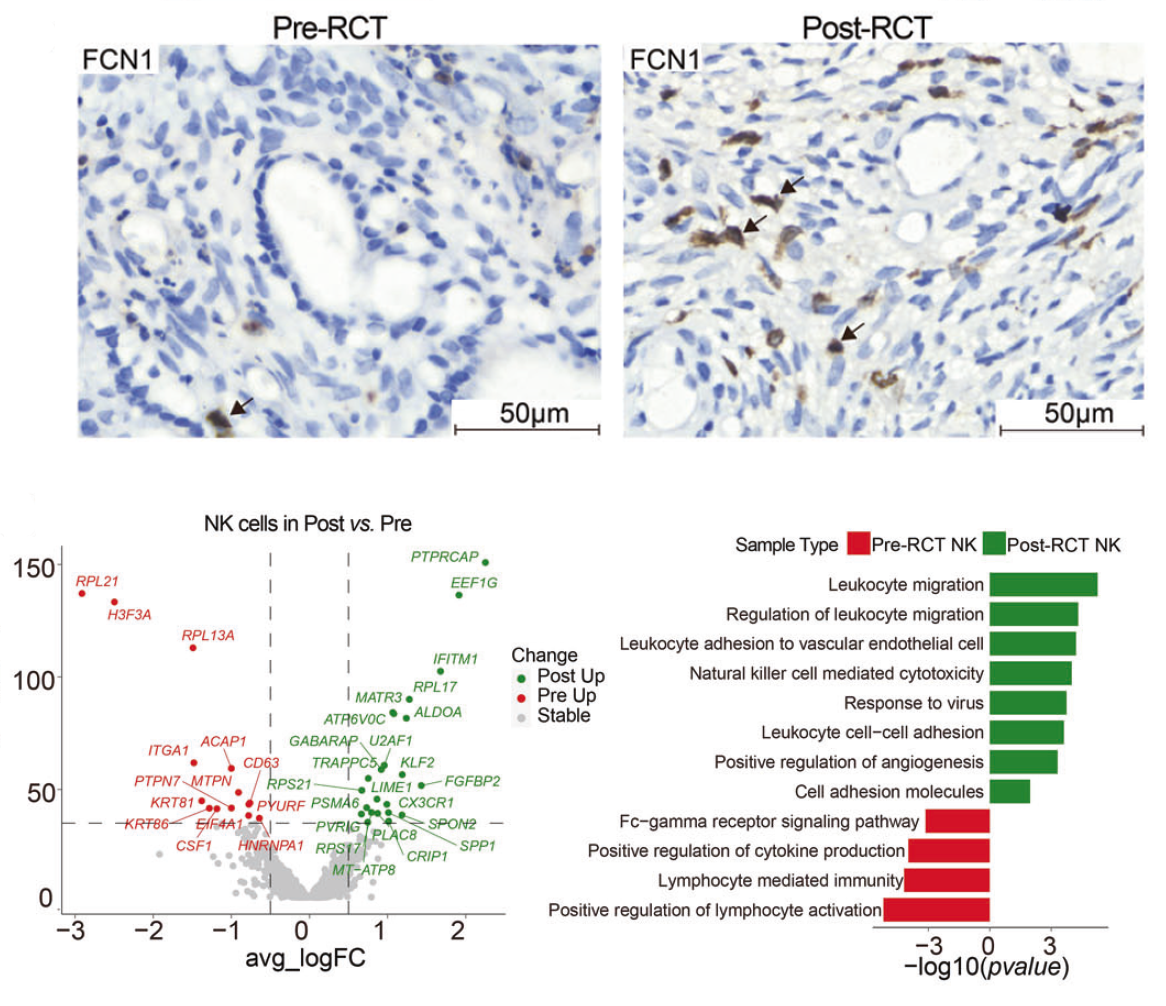
Among lymphocytes, NK and T cells are known for their potential pro- or anti-tumor effects. Ten subclusters of T cells and 2 subclusters of NK cells were identified. CD16_NK and GZMK_CD8 exhibited a clear cytotoxic signature, while Exhausted_CD8 T cells demonstrated high expression of exhaustion marker genes LAG3 and HAVCR2. The proportion of CD16_NK cells significantly increased, as confirmed through IHC staining and CD16 expression analysis. DEG and GO term analyses revealed increased expression of genes associated with leukocyte migration in both NK subpopulations post-RCT and increased expression of cytotoxicity-related genes in the CD16_NK cell subpopulation. CD16_NK cells also exhibited upregulation of cytotoxic granule-associated genes GZMB, GZMH, and NKG7. Furthermore, the expression of these cytotoxic genes positively correlated with patient survival. In contrast, the local T cell population underwent significant restructuring, with an increase in the proportion of CD4_naive cells and a decrease in Exhausted_CD8_T cells. Unlike NK cells, there was no significant increase in cytotoxic properties observed in T cell subclusters during RCT. These findings suggest that CD16+ NK cells are recruited to the cervical cancer tumor microenvironment during RCT, exhibiting increased expression of cytotoxic genes associated with favorable outcomes. On the other hand, T cell subclusters did not show a significant increase in cytotoxic features during RCT treatment. These findings demonstrate the heterogenous effects of RCT on cellular subtypes, which could only be resolved with single-cell RNA-seq.


